Craniofacial Malformations Research Group
The research in our lab aims to identify the underlying, causative molecular and cellular mechanisms of common craniofacial birth defects, with a particular interest in cleft palate and craniosynostosis. We work on the pathogenesis of human mutation using genetic mouse models, in particular those affecting genes in the FGF signalling pathway with a role in bone development and homeostasis. Our main aim is to unravel the molecular events that underlie craniofacial birth defects at the cellular level in order to develop new therapies. Ultimately the hope is that a better understanding of the pathogenesis of these congenital anomalies will translate to improved diagnosis, prognosis and treatment.
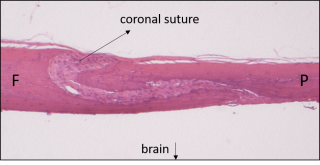
Histological section (H&E) through a normal mouse cranium showing the frontal bone (F) and parietal bone (P) separated by a patent coronal suture.
- Research Projects

Many syndromic forms of craniosynostosis are caused by mutations in genes part of the FGF signalling pathway. In our lab we focus on the FGF receptors and study the effects of mutation of FGFR2 one of the receptor genes. Previous work has shown that FGFR2 is involved in the early pattering of the coronal suture as well as the osteogenesis of neural crest derived calvarial bones.
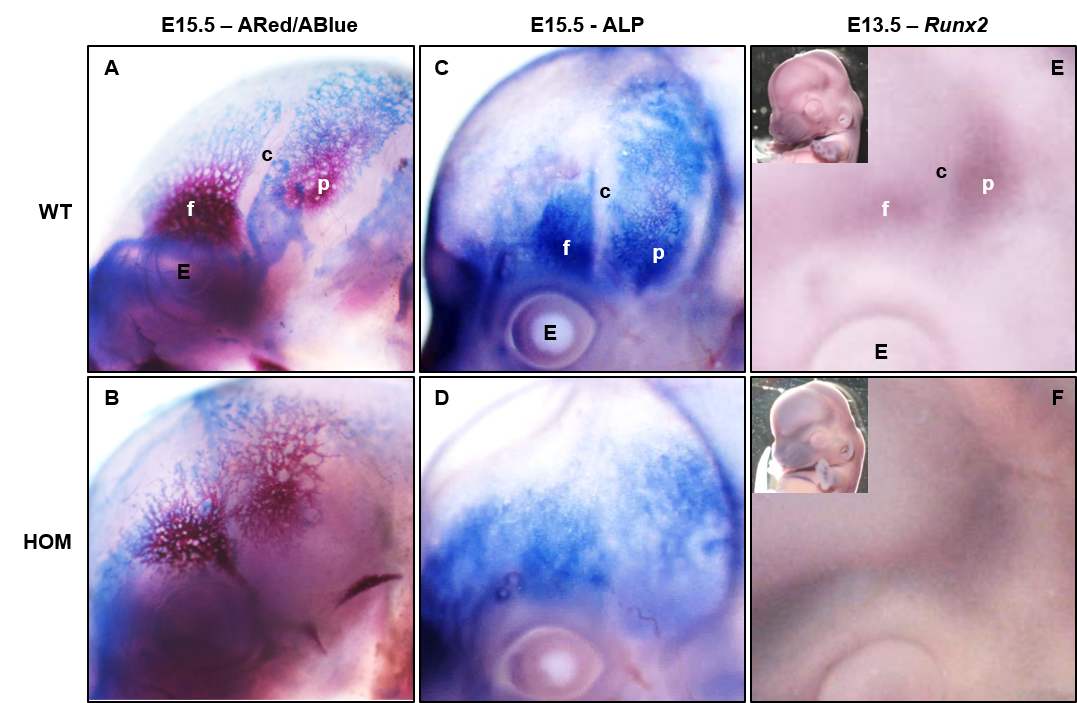
Side view of E15.5 mouse embryos stained with (from left to right) Alizarin Red/Alcian Blue, alkaline phosphatase (ALP) and Runx2. Mutants (HOM) show loss of suture patency. (from Peskett et al. 2017)
This study aims to investigate how external loading of the calvarial bones can delay or prevent the onset and/or progression of craniosynostosis. In addition to the potential translation of this study into clinical treatment we are interested in the cellular and molecular events downstream of calvarial bone mechanotransduction to further understand the underlying mechanism.

3D microCT image of a mouse skull at embryonic day 15.5 showing craniofacial bones
This project -in collaboration with Prof Stanier- explores the relationship between FGF signalling and the embryonic development of the palate. The role of TBX22 -one of the many cleft genes- is also part of this investigation.

- Collaborations
Dr. Mehran Moazen UCL Mechanical Engineering Prof. Phil Stanier UCL Great Ormond Street Institute of Child Health Prof. David Dunaway and Dr. Alessandro Borghi Craniofacial Unit, Great Ormond Street Prof. Andrew Wilkie and Dr. Steve Twigg Weatherall Institute, Oxford Prof. Irene Mathijssen Erasmus University, Rotterdam, Netherlands Dr. Karen Liu Craniofacial Department, King's College London Dr. Maarten Koudstaal Karolinkska Institute, Stockholm, Sweden
- Group Members
Estephania Candelo Gomez
MSc student
Milton Chin
MSc student
Dawn Savery
Research Assistant
Kevin Lee
PhD student
Erwin Pauws
Lecturer/Principal Investigator
Alumni
Emma Peskett
Research Associate
Ivy Richardson
Research Assistant
Samintharaj Kumar
PhD student
Anna Smak-Gregoor
MSc student
Michael Hoffman
MSc student
Nouf Ghaith
MSc student
Liliya Adeeva
MSc student
William Baird
MRes student
Stelia Pissaridou
MSc student
Milad Golsharifi
MSc student
Annette Whittington
MSc student
Janhvi Jaiswal
MSc student
Catherine Webb
MRes student
Renan de Menezes
MRes student
Priyanca Patel
BSc student
Ming Li
MRes student
Sachin Thakrar
MSc student
Jaewon Shin
BSc student
Nihara De Silva
BSc student
Elena Georgidou
MSc student
John Whittingham
MRes student
Maria Iribarren-Guevara
MSc student
Rosanne Aielo
MSc student
Sherine Pranata
BSc student
Charlotte Quinn
MSc student
Somaya Taha
MSc student
- Publications
- Lee, KKL, Stanier P, Pauws E. Mouse models of syndromic craniosynostosis. Molecular Syndromology, 2019.
- Lee KKL, Peskett E, Quinn CM, Aiello R, Adeeva L, Moulding DA, Stanier P, Pauws E. Overexpression of Fgfr2c causes craniofacial bone hypoplasia and ameliorates craniosynostosis in the Crouzon mouse. Disease Models & Mechanisms, 2018.
- Peskett E, Kumar S, Baird W, Jaiswal J, Li M, Patel P, Britto JA, Pauws E. Analysis of the Fgfr2(C342Y) mouse model shows condensation defects due to misregulation of Sox9 expression in prechondrocytic mesenchyme. Biology Open, 2017.
- Twigg SR, Forecki J, Goos JA, Richardson IC, Hoogeboom AJ, van den Ouweland AM, Swagemakers SM, Lequin MH, Van Antwerp D, McGowan SJ, Westbury I, Miller KA, Wall SA; WGS500 Consortium, van der Spek PJ, Mathijssen IM, Pauws E, Merzdorf CS, Wilkie AO. Gain-of-Function Mutations in ZIC1 Are Associated with Coronal Craniosynostosis and Learning Disability. American Journal Human Genetics, 2015.
- Moazen M, Peskett E, Babbs C, Pauws E, Fagan MJ. Mechanical properties of calvarial bones in a mouse model for craniosynostosis. PLoS One, 2015.
- Pauws E, Hoshino A, Bentley L, Prajapati S, Keller C, Hammond P, Martinez-Barbera JP, Moore GE, Stanier P. Tbx22null mice have a submucous cleft palate due to reduced palatal bone formation and also display ankyloglossia and choanal atresia phenotypes. Human Molecular Genetics, 2009.
- Teaching
Module lead for UCL under- and postgraduate teaching:
"Molecular Biology of Normal Development and Birth Defects"
"Genomics, Health and Society"
"Birth Defects: Basic Research and Clinical Applications"
Lecturing at under- and postgraduate level:
BSc Biomedical Sciences, UCL
BSc Population Health, UCL
MBBS, UCL
MSc Paediatrics and Child Health, UCL
MSc Cell and Gene Therapy, UCL
MSc Reproductive and Developmental Biology, Imperial College
Research student supervision at PhD level, together with supervision of laboratory and library projects for MSc, MRes and BSc students at UCL and beyond.
 Close
Close