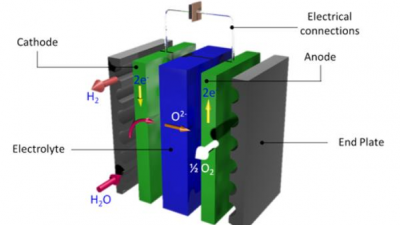
Solid oxide electrolysers effectively operate as reverse fuel cells – they make hydrogen from steam. Solid oxide cells are made of a mixture of ceramics and metals, known as cermets, which can handle the high operating temperatures of between 600 to 1000 °C. These temperatures allow the solid oxide electrolyser to operate more efficiently compared with their low temperature alkaline electrolyser counterpart.
The electrolyser itself works by dissociating steam to hydrogen and oxide ions using electricity at the cathode. The oxide ions are transported through the ion conducting electrolyte to the anode, where the oxide ions join together to form oxygen molecules.
 Close
Close

