Fuel cells are electrochemical energy devices. Meaning? They turn the energy locked in chemicals into electricity directly without any combustion. Because of this they can be much more efficient than other methods of making electricity such as burning fuels to heat water, to make steam, to drive a turbine, to move magnets that induce a current in a wire. A fuel cell turns the fuel straight into electricity with the help of a catalyst – all without any moving parts.
It is very similar to a battery, which also turns chemical energy directly into electricity. The main difference is that while batteries are ‘closed’ (they have all the chemicals stored inside and must be recharged or replaced when they run out) fuel cells are ‘open’ – the fuel is flowed through the device constantly, making electricity as long as the fuel supply lasts.
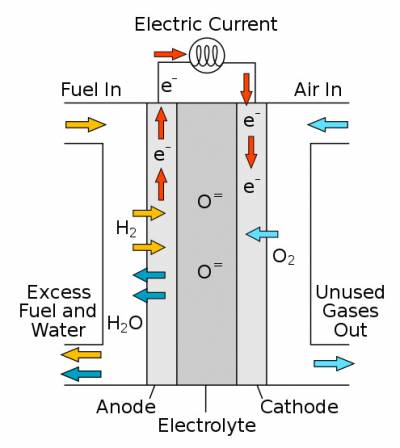
OK, so we have our hydrogen, but what does the fuel cell do to turn it into electricity, and how? A fuel cell is made of 3 main parts; an anode, a cathode and an electrolyte. The anode and cathode are coated with a catalyst that helps the reactions happen.
Anode: This is the negative end of the fuel cell and where the hydrogen is split into its protons and electrons. The protons travel through the electrolyte and the electrons go around the circuit, powering whatever is plugged in to the fuel cell.
Electrolyte: The electrolyte is a special polymer plastic membrane that allows positively charged protons to travel through it, but blocks electrons. It’s important to electrically insulate the anode from the cathode otherwise you get a short circuit.
Cathode: Once the protons have travelled through the electrolyte they combine at the cathode with the electrons (after they have been through the circuit and done their work) and the oxygen to make water, which is removed in the fuel cell exhaust.
Catalyst: When the hydrogen and the oxygen react, there is an energy barrier that they must overcome before they are able to combine – like a hill that you must get over before being able to roll down the other side. A catalyst works to reduce the height of this hill and so make the hydrogen and oxygen react more easily. Without it, they would not react at all. Most fuel cell catalysts are made from nanoparticles of platinum metal, which can be a source of high cost.
How Big Are Fuel Cells?
One of the benefits of fuel cells is that they are very scalable. The power you get out of a fuel cell is directly proportional to the area of the cell, and many individual cells can be connected together to make what is called a stack. Because of this they have potential uses for many devices from phones and laptops to cars and power for houses – even as power stations in their own right.
Advantages:
- Efficient
- Quiet
- No pollution at point of use
- Portable
- Scalable
- No moving parts
Disadvantages:
- Expensive
- Durability problems
- Infrastructure – there would need to be a hydrogen network in order to, say, fill up a car easily.
 Close
Close

