New genetics discovery paves the way to transform immunotherapy treatments
11 March 2016
An international team of scientists, led by Dr Sergio Quezada and Professor Charles Swanton, have made a groundbreaking discovery in understanding how the genetic complexity of cancerous tumours can be recognised and exploited by the immune system, even when the disease is at its most advanced stages.
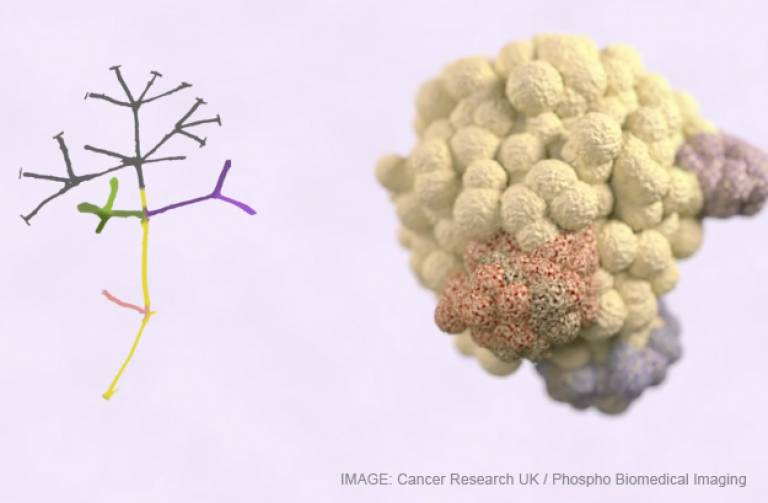
The landmark findings published in Science could guide future immunotherapies and improve the way existing drugs are used. Leading on from the study, researchers hope to launch the first human trial in lung cancer patients in the next two years.
Immunotherapy is currently one of the most promising areas of cancer research. In recent years, the field has made great progress in developing ways to harness the immune system to fight cancers. When treatments work, they work extremely well. However at present, immunotherapy treatments only work for a limited range of cancer types and even then, the number of patients who respond to treatment is in the minority.
To try and understand this why this might be, lead authors Dr Sergio Quezada, Head of the Immune Regulation and Cancer Immunotherapy lab at UCL Cancer Institute, and Professor Charles Swanton, the Francis Crick Institute, Chair in Personalised Cancer Medicine at UCL Cancer Institute and Consultant Medical Oncologist at UCLH, joined forces to investigate how the immune system recognises cancer tumours, and, what it is that is being recognised.
Tumour evolution
As a tumour develops, the diversity of its genetic faults can be flagged on the cancer cell surface, as unique mutations appear in different parts of the tumour.
Crucially, by analysing data from hundreds of patients from previous studies, researchers found that some of these flags - known as antigens - represent the very earliest mutations of the disease and are common ie displayed on all cells in the tumour, rather than a subset of tumour cells.
In the laboratory, specialised immune cells, called T-cells, were isolated from samples of two patients with lung cancer. These isolated T-cells could recognise these common flags present on every tumour cell. Even though these potent immune cells have the potential to wipe-out all cancerous cells within the tumour, they are switched off by the tumour's defence mechanisms.
This study provides the first evidence that there are seeds of a tumour's own destruction within the tumour itself and paves the way for therapies that specifically activate these T-cells to target all the tumour cells at once based on the disease's genetic signature. In the future, scientists could exploit this by developing a therapeutic vaccine to activate T-cells, or harvesting, growing and administering T-cells back into the patient that recognise the antigens common to every cancer cell.
Targeting treatment
"The body's immune system acts as the police in trying to tackle cancer, the criminals. Genetically diverse tumours are like a gang of hoodlums involved in different crimes - from robbery to smuggling. And the immune system struggles to keep on top of the cancer - just as it it's difficult for police when there's so much going on."
"Our research shows that instead of aimlessly chasing crimes in different neighbourhoods, we can give the police the information they need to get to the kingpin at the root of all organised crime - the weak spot in the patient's tumour - to wipe out the problem for good." explains Dr Quezada.
The genetic complexity of cancer, which is flagged by tumour antigens, arises when cancers evolve in a branched manner. The earliest faults are founds in all cells. forming the 'trunk' of the disease, while the later mutations arise in some cells but not all. It is these 'branches' that allow the disease to adapt and become drug resistant.
Professor Charles Swanton says "This is exciting. There was evidence that complex tumours with many mutations could increase the chance of the immune system spotting them; now we can prioritise and target tumour antigens that are present in every cell, the Achilles heel of these highly complex cancers".
"This opens up a way to look at individual patients' tumours and profile all the antigen variations to figure out the best ways for immunotherapy treatments to work, prioritising antigens present in every tumour cell and identifying the body's immune T-cells that recognise them. This is really fascinating, and takes personalised medicine to its absolute limit where each patient would have a unique, bespoke treatment."
Dr Quezada adds, "For many years we have studied how the immune response to cancer is regulated without a clear understanding of what it is that immune cells recognise on cancerous cells. Based on these new findings, we will be able to tell the immune system how to specifically recognise and attack tumours."
Related news
Accelerating Immunotherapy with £5m Award
Cancer Research UK has awarded a £5 million Centres Network Accelerator Award to UCL’s Cancer Research UK Centre to fund a new cancer immunology and immunotherapy network across various Cancer Centres in London - led by Professor Henning Walczak, Scientific Director of the Cancer Research UK – UCL Centre and Professor of Cancer Biology at the UCL Cancer Institute.
Further information:
- Research paper: 'Clonal neoantigens elicit T cell immunoreactivity and sensitivity to immune checkpoint blockade' Science
- Dr Sergio Quezada academic profile
- Professor Charles Swanton academic profile
- UCL Faculty of Medical Sciences
- The Francis Crick Institute
- Cancer Research-UK UCL Centre
- Image: with thanks to Cancer Research UK and Phospho Biomedical Imaging
- Source: Cancer Research UK
The research was supported by the National Institute for Health Research Biomedical Research Centre at University College London Hospitals NHS Foundation Trust and University College London, Cancer Research UK and the Rosetrees Trust and involved researchers from UCL, Harvard and Massachusetts Institute of Technology.
 Close
Close