The Patel lab: Ca2+ signalling through acidic organelles
Our lab focuses on the role of acidic organelles, such as lysosomes, in Ca2+ signalling in both health and disease. See the lay section for a plain English summary.
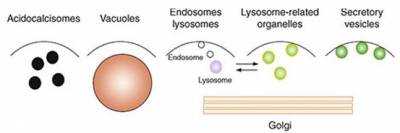
Background
Changes in cytosolic Ca2+ form the basis of a ubiquitous, evolutionary conserved signalling pathway. These Ca2+ signals drive many, if not all cellular processes from the very start of life (fertilisation), during vital processes such as neurotransmission, through to death (apoptosis). It is now clear that so-called "acidic Ca2+ stores" dynamically regulate cytosolic Ca2+ levels, both in isolation and in conjunction with the better-characterised Ca2+ stores of the endoplasmic reticulum. Acidic Ca2+ stores is an umbrella term that describes a cross-kingdom collection of morphologically distinct-yet-functionally related organelles united by their acid and Ca2+-rich interior. Lysosomes and lysosome-related organelles are key acidic Ca2+ stores that are considered functionally related to acidocalcisomes, vacuoles, endosomes, secretory granules and the Golgi complex. The Ca2+ mobilising messenger NAADP plays a critical role in controlling acidic Ca2+ store signalling.
Vision
We are i) defining the Ca2+ signalling "toolkit" (channels, transporters) of acidic Ca2+ stores at the molecular level and their evolutionary origins (form), ii) probing their physiological roles in processes such as membrane trafficking and cell migration (function) and iii) investigating how deviant signalling through these organelles can precipitate disorders such as Parkinson disease (failure).
Approach
Our work is interdisciplinary, encompassing both "wet" (molecular, cell and structural biology) and "dry" (bioinformatics, phylogenetics and computational modelling) approaches in a highly collaborative and international context.
NAADP: An unusual Ca2+ mobilizing messenger
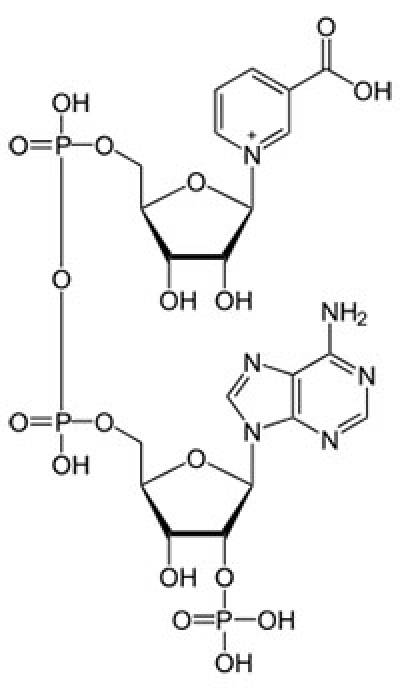
NAADP is a potent Ca2+ mobilising messenger, discovered in the 1990s by Hon Cheung Lee and colleagues. Over the last 15 years or so, we have played a central role in defining its mechanism of action using many model systems spanning from sea urchin eggs to primary human fibroblasts. In early work, we: i) identified and characterised the binding properties of NAADP receptors; ii) showed that NAADP is highly unusual in activating Ca2+-permeable channels located on non-canonical, acidic Ca2+ stores such as lysosomes and lysosome-like organelles; iii) quantified cellular NAADP levels and identified NAADP generating signals, such as the hormones and neurotransmitters; iv) identified and characterised NAADP synthesizing enzymes - the ADP-ribosyl cyclases - from both invertebrate and vertebrate systems and v) extended the actions of NAADP to the nervous system.
Patel, S., G.C.Churchill, T.Sharp, and A.Galione. 2000. Widespread distribution of binding sites for the novel Ca2+-mobilizing messenger, nicotinic acid adenine dinucleotide phosphate, in the brain. J. Biol. Chem. 275:36495-36497.
Berridge, G., G.Dickinson, J.Parrington, A.Galione, and S.Patel. 2002. Solubilization of receptors for the novel Ca2+-mobilizing messenger, nicotinic acid adenine dinucleotide phosphate. J. Biol. Chem. 277:43717-43723.
Churchill, G.C., Y.Okada, J.M.Thomas, A.A.Genazzani, S.Patel, and A.Galione. 2002. NAADP mobilizes Ca2+ from reserve granules, lysosome-related organelles, in sea urchin eggs. Cell 111:703-708.
Churamani, D., E.A.Carrey, G.D.Dickinson, and S.Patel. 2004. Determination of cellular nicotinic acid-adenine dinucleotide phosphate (NAADP) levels. Biochem. J. 380:449-454.
Yamasaki, M., J.T.Thomas, G.C.Churchill, C.Garnham, A.L.Lewis, J.M.Cancela, S.Patel, and A.Galione. 2005. Role of NAADP and cADPR in the induction and maintenance of agonist-evoked Ca2+ spiking in mouse pancreatic acinar cells. Curr. Biol. 15:874-878.
Brailoiu, E., J.L.Hoard, C.M.Filipeanu, G.C.Brailoiu, S.L.Dun, S.Patel, and N.J.Dun. 2005. Nicotinic acid adenine dinucleotide phosphate potentiates neurite outgrowth. J. Biol. Chem. 280:5646-5650.
Churamani, D., M.J.Boulware, T.J.Geach, A.C.Martin, G.W.Moy, Y.H.Su, V.D.Vacquier, J.S.Marchant, L.Dale, and S.Patel. 2007. Molecular characterization of a novel intracellular ADP-ribosyl cyclase. PLoS. ONE. 2:e797.
Pandey, V., C.C.Chuang, A.M.Lewis, P.Aley, E.Brailoiu, N.Dun, G.C.Churchill, and S.Patel. 2009. Recruitment of NAADP-sensitive acidic Ca2+ stores by glutamate. Biochem. J. 422:503-512.
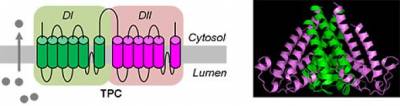
The two-pore channels (TPCs)
In 2009, we were one of three groups that converged on the TPCs as the long-sought-after target channels for NAADP. TPCs have a duplicated domain architecture comprising two concatenated voltage-gated ion channel-like repeats, each containing 6 transmembrane regions and a re-entrant pore loop. They likely dimerise to form the characteristic pseudotetrameric arrangement typical of the voltage-gated ion channel superfamily to which TPCs belong. We showed that TPCs localise to the endolysosomal system and applied loss- and gain-of function approaches, together with Ca2+ imaging and electrophysiology (with Rahman and Muallem), to show that TPCs are likely the pore-forming subunit of NAADP-gated, Ca2+-permeable channels. Our work with Marchant suggests that NAADP does not bind directly to TPCs, but instead to closely associated NAADP-binding protein(s). Modifiers of voltage-gated Ca2+ and Na+ channels also block TPCs, possibly though a common binding site. We are currently performing structure-function analyses of TPCs.
Brailoiu, E., D.Churamani, X.Cai, M.G.Schrlau, G.C.Brailoiu, X.Gao, R.Hooper, M.J.Boulware, N.J.Dun, J.S.Marchant, and S.Patel. 2009. Essential requirement for two-pore channel 1 in NAADP-mediated calcium signaling. J. Cell Biol. 186:201-209.
Brailoiu, E., T.Rahman, D.Churamani, D.L.Prole, G.C.Brailoiu, R.Hooper, C.W.Taylor, and S.Patel. 2010b. An NAADP-gated two-pore channel targeted to the plasma membrane uncouples triggering from amplifying Ca2+ signals. J. Biol. Chem. 285:38511-38516.
Hooper, R., D.Churamani, E.Brailoiu, C.W.Taylor, and S.Patel. 2011. Membrane topology of NAADP-sensitive two-pore channels and their regulation by N-linked glycosylation. J. Biol. Chem. 286:9141-9149.
Churamani, D., R.Hooper, E.Brailoiu, and S.Patel. 2012. Domain assembly of NAADP-gated two-pore channels. Biochem. J. 441:317-323.
Lin-Moshier, Y., T.F.Walseth, D.Churamani, S.M.Davidson, J.T.Slama, R.Hooper, E.Brailoiu, S.Patel, and J.S.Marchant. 2012. Photoaffinity labeling of nicotinic acid adenine dinucleotide phosphate (NAADP) targets in mammalian cells. J. Biol. Chem. 287:2296-2307.
Jha, A., M.Ahuja, S.Patel, E.Brailoiu, and S.Muallem. 2014. Convergent Regulation of the Lysosomal Two-Pore Channel-2 by Mg2+, NAADP, PI(3,5)P2 and Multiple Protein Kinases. EMBO J. 33:501-511
Jha, A., M.Ahuja, S.Patel, E.Brailoiu, and S.Muallem. 2014. Convergent Regulation of the Lysosomal Two-Pore Channel-2 by Mg2+, NAADP, PI(3,5)P2 and Multiple Protein Kinases. EMBO J. 33:501-511
Rahman, T., X.Cai, G.C.Brailoiu, M.E.Abood, E.Brailoiu, and S.Patel. 2014. Two-pore channels provide insight into the evolution of voltage-gated Ca2+ and Na+ channels. Sci. Signal. 7:ra109.
Patel, S. 2015. Function and dysfunction of two-pore channels. Sci. Signal. 8:re7.
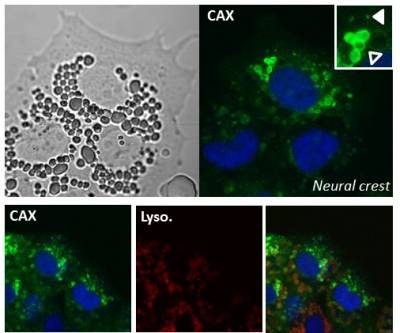
Ca2+/H+ exchangers (CAX)
How does Ca2+ get into acidic organelles? In plants and protist vacuoles, Ca2+ uptake is mediated in part by Ca2+-H+ exchangers (CAX). In 2016, we identified CAXs in animals and shown that they localize to acidic Ca2+ stores to mediate Ca2+ filling.
Melchionda,M., Pittman,J.K., Mayor,R., & Patel,S. (2016) Ca2+/H+ exchange by acidic organelles regulates cell migration in vivo. J. Cell Biol. 212, 803-813.
Inter-organellar crosstalk
NAADP is often described as "trigger" for Ca2+ signalling. This is because the Ca2+ signals it evokes from acidic organelles can be amplified by neighbouring channels on the ER, likely through the process of Ca2+-induced Ca2+-release. We have proposed that such "chatter" occurs at membrane contact sites between the ER and the endolysosomal system. Membrane contact sites are regions of close apposition where different membranes come in close contact (<30 nm) but do not fuse. With Futter, we have identified membrane contact sites between the lysosomes and ER. Computational modelling with Sneyd has provided insight into Ca2+ signalling at these junctions. Our recent work shows that Ca2+ released from endosomes serves to strengthen contact with the ER.
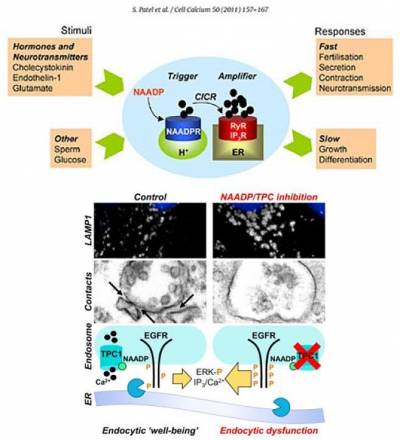
Patel, S., and E.Brailoiu. 2012. Triggering of Ca2+ signals by NAADP-gated two-pore channels. A role for membrane contact sites? Biochem. Soc. Trans. 40:153-157.
Kilpatrick, B.S., E.R.Eden, A.H.Schapira, C.E.Futter, and S.Patel. 2013. Direct mobilisation of lysosomal Ca2+ triggers complex Ca2+ signals. J. Cell Sci. 126:60-66.
Penny, C.J., B.S.Kilpatrick, J.M.Han, J.Sneyd, and S.Patel. 2014. A computational model of lysosome-ER Ca2+ microdomains. J. Cell Sci. 127:2934-2943.
Penny, C. J., Kilpatrick, B. S., Eden, E. R., and Patel, S. (2015) Coupling acidic organelles with the ER through Ca microdomains at membrane contact sites Cell Calcium 58, 387-96.
Kilpatrick,B.S., Eden,E.R., Hockey,L.N., Yates,E., Futter,C.E., & Patel,S. (2017) An Endosomal NAADP-Sensitive Two-Pore Ca2+ Channel Regulates ER-Endosome Membrane Contact Sites to Control Growth Factor Signaling. Cell Rep. 18, 1636-1645.
Membrane trafficking and cell migration
It is likely that the local Ca2+ signals derived from acidic organelles might act in their own right to drive physiological processes. Indeed, it has been known for some time that local Ca2+ signals regulate various trafficking events within the endolysosomal system. With Marchant, we have shown that TPCs associate with a variety of proteins involved in membrane trafficking (such as Rab GTPases) to regulate endolysosomal morphology. With Mayor, we have linked CAX to migration of neural crest cell cells. We are currently defining the mechanisms by which TPCs and CAX regulate subcellular and cellular motility.
Lin-Moshier, Y., M.V.Keebler, R.Hooper, M.J.Boulware, X.Liu, D.Churamani, M.E.Abood, T.F.Walseth, E.Brailoiu, S.Patel, and J.S.Marchant. 2014. The two-pore channel (TPC) interactome unmasks isoform-specific roles for TPCs in endolysosomal morphology and cell pigmentation. Proc. Natl. Acad. Sci. 111:13087-13092.
Melchionda,M., Pittman,J.K., Mayor,R., & Patel,S. (2016) Ca2+/H+ exchange by acidic organelles regulates cell migration in vivo. J. Cell Biol. 212, 803-813.
Parkinson disease
There is an increasing appreciation of the role of lysosomal dysfunction in neurodegenerative disorders such as Alzheimer's and Parkinson disease. With Schapira, we have recently identified both endolysosomal membrane trafficking and Ca2+ defects in fibroblasts from Parkinson disease patients. We showed that chemical or molecular inhibition of the NAADP pathway restores endocytic "well-being" in LRRK2 Parkinson disease. We are currently exploring whether the NAADP-signalling pathway might therefore represent a novel therapeutic target in Parkinson disease.
Hockey, L. N., Kilpatrick, B. S., Eden, E. R., Lin-Moshier, Y., Brailoiu, G. C., Brailoiu, E., Futter, C., Schapira, A. H., Marchant, J. S. and Patel, S. (2015) Dysregulation of lysosomal morphology by pathogenic LRRK2 is corrected by two-pore channel 2 inhibition. J.Cell Sci. 128, 232-238
Kilpatrick,B.S., Magalhaes,J., Beavan,M.S., McNeill,A., Gegg,M.E., Cleeter,M.W., Bloor-Young,D., Churchill,G.C., Duchen,M.R., Schapira,A.H. and Patel,S. (2016) Endoplasmic reticulum and lysosomal Ca2+ stores are remodelled in GBA1-linked Parkinson disease patient fibroblasts. Cell Calcium. 59, 12-20
Evolution
Despite their diversity, acidic Ca2+ stores may function similarly across the natural world in processes such as signalling, membrane traffic and secretion. TPCs are particularly interesting in an evolutionary context in terms of their phylogenomic profile (i.e. the presence of their genes in specific organisms) and their putative structure. Most deuterostome animals possess three TPC isoforms but there is highly unusual, lineage-specific loss of TPC3 in certain primates (including humans) and rodents. Their duplicated domain organisation indicates that they may be descendants of the key evolutionary intermediate between one- and four-domain members of the voltage-gated ion channel superfamily. With Cai, we have established the phylogenetic relationship between multi-domain voltage-gated ion channels, and recently identified a novel clade of TPCs in unicellular organisms with predicted properties similar to voltage-gated Ca2+ channels. We are exploring the evolutionary trajectory for TPCs and their related channels.
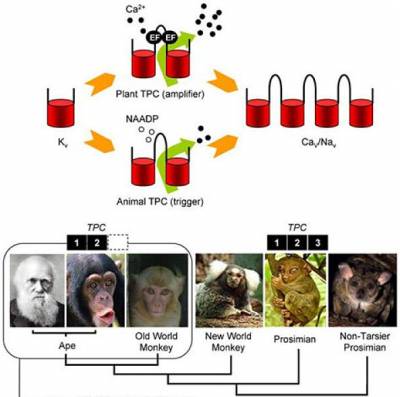
Patel, S., and R.Docampo. 2010. Acidic calcium stores open for business: expanding the potential for intracellular Ca2+ signaling. Trends Cell Biol. 20:277-286.
Brailoiu, E., R.Hooper, X.Cai, G.C.Brailoiu, M.V.Keebler, N.J.Dun, J.S.Marchant, and S.Patel. 2010. An ancestral deuterostome family of two-pore channels mediate nicotinic acid adenine dinucleotide phosphate-dependent calcium release from acidic organelles. J. Biol. Chem. 285:2897-2901.
Cai, X., and S.Patel. 2010. Degeneration of an intracellular ion channel in the primate lineage by relaxation of selective constraints. Mol. Biol. Evol. 27:2352-2359.
Patel, S., and S.Muallem. 2011. Acidic Ca2+ stores come to the fore. Cell Calcium 50:109-112.
Rahman, T., X.Cai, G.C.Brailoiu, M.E.Abood, E.Brailoiu, and S.Patel. 2014. Two-pore channels provide insight into the evolution of voltage-gated Ca2+ and Na+ channels. Sci. Signal. 7:ra109.
Patel, S. and Cai, X. (2015) Evolution of acid Ca2+ stores and their resident Ca2+-permeable channels Cell Calcium 57, 222-230.
Sandip Patel

Principal investigator
Sandip Patel obtained a BSc in Medical Biochemistry from the University of Birmingham in 1992, and a PhD in Pharmacology from Cambridge University in 1995 under the supervision of Prof. Colin W. Taylor.
He was awarded a Wellcome Trust "Prize" International Travel Fellowship in 1996. This allowed him to do post-doctoral work with Prof. Andrew P. Thomas, initially at Thomas Jefferson University and then at the University of Medicine and Dentistry of New Jersey (now Rutgers University) in the USA (1996-1998). He spent the last year of his fellowship (1999-2000) with Prof Antony Galione at Oxford University where Sandip developed his interests in NAADP.
Sandip was awarded a Wellcome Trust Career Development Fellowship in 2000 which allowed him to set up his own lab at Oxford. He also held the Hayward Junior Research Fellowship at Oriel College at this time. In 2001, he re-located the lab to University College London and was awarded a lectureship. Sandip was promoted to Reader in 2007 and Professor in 2011.
Sandip was Vice-chair of the Signalling Panel of Biochemical Society (2009-2012) and currently a member of the Grants advisory panel of Parkinson's UK (2016-). He is an associate Editor of Messenger (2012-) and a member of the Editorial boards of Biology of the Cell (2003-2006), the Biochemical Journal (2004-2011), Cell Calcium (2007-) and Contact (2018-).
He was elected a Fellow of the Royal Society of Biology (FRSB) in 2013.
Stephen Bolsover

Emeritus Professor and Researcher
Martina Gregori

PhD student
Rebecca Jones

PhD student
Bethan S. Kilpatrick
Post doc
Kristin Vassileva (Primary Supervisor: Mark Marsh)

PhD student
Yu (Cara) Yuan

PhD student
Alumni
Harry Ward (MSc student, 2017 - 2018)
Adam Fitchett (MSc student, 2016 - 2017)
Stephen Mullin (PhD student with Anthony Schapira, 2014 - 2017)
Elizabeth Yates (PhD student, 2013 - 2017)
Manuela Melchionda (Post doc, 2013 - 2016)
Christopher Penny (PhD student, 2013 - 2016)
Leanne Hockey (MSc student, 2012 - 2013)
Bethan Kilpatrick (PhD student, 2011 - 2014)
Suchita Deo (MSc student, 2010 - 2011)
Bethan Kilpatrick (MSc student, 2010 - 2011)
George Dickinson (Post doc, 2008 - 2010)
Robert Hooper (PhD student, 2008 - 2011)
Latha Ramakrishnan (PhD student, 2007 - 2010)
Dev Churamani (Post doc, 2006 - 2012)
Vinita Pandey (Post doc, 2004 - 2006)
Dev Churamani (PhD student, 2002 - 2005)
George Dickinson (Post doc, 2002 - 2004)
Dayle Hogg (Post doc, 2000 - 2001)
Georgina Berridge (Research assistant, 2000 - 2001)
Our lab collaborates extensively at the local, national and international level.
| University College London | |
| Leslie Dale | |
| Michael R. Duchen | |
| Sean M. Davidson | |
| Clare E. Futter | |
| Mark Marsh | |
| Andrew Martin | |
| Roberto Mayor | |
| Sara Mole | |
| Anthony H. Schapira |
| UK | |
| Bonnie A. Wallace | Birkbeck College |
| Grant C. Churchill | Oxford University |
| Jon Pittman | Manchester University |
| Taufiq Rahman | Cambridge University |
| International | |
| Teresa Alonso | Valladolid University, Spain |
| Eugen Brailoiu | Temple University, USA |
| Xinjiang Cai | Rutgers University, USA |
| Christian Grimm | Ludwig-Maximilians-Universität, Germany |
| Sabine Hilfiker | CSIC, Spain |
| Karl-Johan Malmberg | University of Oslo, Norway |
| Jonathan Marchant | University of Minnesota, USA |
| Silvia Moreno | University of Georgia, USA |
| Shmuel Muallem | NIH, USA |
| Israel Sekler | Ben-Gurion University of the Negev, Israel |
| Soraya Smaili | Federal University of São Paulo, Brazil |
| James Sneyd | University of Auckland, New Zealand |
| Mathias Ziegler | University of Bergen, Norway |
Our published work is regularly highlighted or attracts commentaries by our peers.
Kilpatrick,B.S., Eden,E.R., Hockey,L.N., Yates,E., Futter,C.E., & Patel,S. (2017) An Endosomal NAADP-Sensitive Two-Pore Ca2+ Channel Regulates ER-Endosome Membrane Contact Sites to Control Growth Factor Signaling. Cell Rep. 18, 1636-1645.

Commentary by Docampo R
Melchionda,M., Pittman,J.K., Mayor,R., & Patel,S. (2016) Ca2+/H+ exchange by acidic organelles regulates cell migration in vivo. J. Cell Biol. 212, 803-813.
Lloyd-Evans,E. (2016) On the move, lysosomal CAX drives Ca2+ transport and motility. J. Cell Biol. 212, 755-757.
Penny,C.J., Rahman,T., Sula,A., Miles,A.J., Wallace,B.A., & Patel,S. (2016) Isolated pores dissected from human two-pore channel 2 are functional. Sci. Rep. 6, 38426.

Commentary by Galione A and Morgan A
Davidson,S.M., Foote,K., Kunuthur,S., Gosain,R., Tan,N., Tyser,R., Zhao,Y.J., Graeff,R., Ganesan,A., Duchen,M.R., Patel,S. and Yellon,D.M. (2015) Inhibition of NAADP signalling on reperfusion protects the heart by preventing lethal calcium oscillations via two-pore channel 1 and opening of the mitochondrial permeability transition pore. Cardiovasc. Res. 108, 357-366.
Commentary: Tsukamoto, O., H.Asanuma, and M.Kitakaze. (2015). Targeting lysosomal Ca2+ to reduce reperfusion injury. Cardiovasc. Res. 108, 321-323.
Hockey, L. N., Kilpatrick, B. S., Eden, E. R., Lin-Moshier, Y., Brailoiu, G. C., Brailoiu, E., Futter, C., Schapira, A. H., Marchant, J. S. and Patel, S. (2015) Dysregulation of lysosomal morphology by pathogenic LRRK2 is corrected by two-pore channel 2 inhibition. J.Cell Sci. 128, 232-238
Highlighted as an "In This Issue" article. "Two-pore channels in Parkinson disease"
Rahman, T., Cai, X., Brailoiu, G. C., Abood, M. E., Brailoiu, E. and Patel, S. (2014) Two-pore channels provide insight into the evolution of voltage-gated Ca2+ and Na+ channels. Sci.Signal. 7, ra109
Features on front cover. Commentary: Adler, E. M. (2015) Of ELIC and evolution J.Gen.Physiol 145, 1-2
Penny, C. J., Kilpatrick, B. S., Han, J. M., Sneyd, J. and Patel, S. (2014) A computational model of lysosome-ER Ca2+ microdomains. J.Cell Sci. 127, 2934-2943

Commentary by Dupont G
Jha, A., Ahuja, M., Patel, S., Brailoiu, E. and Muallem, S. (2014) Convergent Regulation of the Lysosomal Two-Pore Channel-2 by Mg2+, NAADP, PI(3,5)P2 and Multiple Protein Kinases. EMBO J. 33, 501-511

Commentary by Tepikin A
Churamani, D., Hooper, R., Rahman, T., Brailoiu, E. and Patel, S. (2013) The N-terminal region of two-pore channel 1 regulates trafficking and activation by NAADP. Biochem.J. 453, 147-151

Commentary by Tepikin A

Commentary by Docampo R
Commentary: Guse, A. H. (2013) N-terminal tagging of two-pore channels interferes with NAADP action Biochem.J. 453, e1-e2
Kilpatrick, B. S., Eden, E. R., Schapira, A. H., Futter, C. E. and Patel, S. (2013) Direct mobilisation of lysosomal Ca2+ triggers complex Ca2+ signals. J.Cell Sci. 126, 60-66

Commentary by Petersen O
Lin-Moshier, Y., Walseth, T. F., Churamani, D., Davidson, S. M., Slama, J. T., Hooper, R., Brailoiu, E., Patel, S. and Marchant, J. S. (2012) Photoaffinity labeling of nicotinic acid adenine dinucleotide phosphate (NAADP) targets in mammalian cells. J.Biol.Chem. 287, 2296-2307
Paper of the week. Commentary: Guse, A. H. (2012) Linking NAADP to Ion Channel Activity: A Unifying Hypothesis Sci.Signal. 5, e18
Brailoiu, E., Churamani, D., Cai, X., Schrlau, M. G., Brailoiu, G. C., Gao, X., Hooper, R., Boulware, M. J., Dun, N. J., Marchant, J. S. and Patel, S. (2009) Essential requirement for two-pore channel 1 in NAADP-mediated calcium signaling. J.Cell Biol. 186, 201-209
Highlighted as an "In This Issue" article. "Calcium's route to freedom"
Brailoiu, E., Churamani, D., Pandey, V., Brailoiu, G. C., Tuluc, F., Patel, S. and Dun, N. J. (2006) Messenger-specific role for NAADP in neuronal differentiation. J.Biol.Chem. 281, 15923-15928

Commentary by Galione A
Brailoiu, E., Hoard, J. L., Filipeanu, C. M., Brailoiu, G. C., Dun, S. L., Patel, S. and Dun, N. J. (2005) NAADP potentiates neurite outgrowth. J.Biol.Chem. 280, 5646-5650

Commentary by Galione A
Brailoiu, E., Patel, S. and Dun, N. J. (2003) Modulation of spontaneous transmitter release from the frog neuromuscular junction by interacting intracellular Ca2+ stores: critical role for nicotinic acid-adenine dinucleotide phosphate (NAADP). Biochem.J. 373, 313-318
Commentary: Rutter, G. A. (2003) Calcium signalling: NAADP comes out of the shadows Biochem.J. 373, e3-e4
Patel, S., Robb-Gaspers, L. D., Stellato, K. A., Shon, M. and Thomas, A. P. (1999) Coordination of calcium signalling by endothelial-derived nitric oxide in the intact liver. Nat.Cell Biol. 1, 467-471
Commentary: Charles, A. (1999) Nitric oxide pumps up calcium signalling Nat.Cell Biol. 1, E193-E195 (News and Views)
Commentary: Tordjmann, T., Combettes, L., and Claret, M. (2000) Nitric oxide as a calcium wave accelerator Hepatology 32, 156-157
Mission
Calcium increases within our cells drive just about all cellular processes. Indeed, when these increases go awry, diseases develop including those that affect the brain. Therefore, calcium is not just important for bones and teeth!
Our work over the last 15 years has shown that tiny structures within our cells known as lysosomes are important for regulating calcium levels within cells.
Our mission is to identify the proteins that allow lysosomes to handle calcium, to understand the role of lysosome calcium in regulating aspects of cell behaviour and whether mis-handling of calcium by lysosomes is responsible for disease.
 |  |  |
Calcium and Parkinson disease (funded by Parkinson's UK)
There is mounting evidence to suggest that defects in lysosomes may contribute to Parkinson disease. Parkinson disease is a common neurodegenerative disorder that affects the midbrain resulting in characteristic tremors. Lysosomes are best known for recycling waste material but we think that calcium within the lysosomes might be important in disease progression.
Click here for a lay article in the Atlas of Science.
Calcium and cell migration (funded by BBSRC)
One of the many functions ascribed to calcium is the process of cell migration. Cells often move (migrate) in a specific direction to a given location in response to external signals. This is particularly important during embryo development. Defective migration underlies a range of disorders including cancer. We are investigating the role of calcium handling by lysosomes in the migration of neural crest cells. Neural crest cells are found in the embryo and migrate throughout the developing organism to give rise to a range of other cells/tissues including pigmented cells and parts of the nervous system.
 Close
Close




