AUC can be applied to a wide range of biological systems. An analytical ultracentrifuge identifies the solution properties of macromolecules (including their interactions), facilitates solution structural determinations of large proteins using atomistic modelling, and complements new NMR and crystal structure determinations.
We possess a new Optima multiwavelength AUC instrument (delivered 2019; the first one to be installed at a UK university) and two older XL-I AUC instruments, all from Beckman-Coulter, and all equipped with four-hole and eight-hole rotors and six-sector equilibrium cells and two-sector velocity cells. One XL-I is equipped with fluorescent optics. Samples are studied using absorbance, interference and/or fluorescent optics.
Two types of experiments are generally performed:
(1) In sedimentation velocity experiments, if the sample is subjected to high speed rotor speeds of between 30-60k rpm, relatively rapid sedimentation of the sample towards the bottom of the cell occurs. From SEDFIT analyses of the sedimentation boundsaries, the sedimentation coefficient (s20,w) provides structural data for comparison with crystallography, scattering and NMR. Multiple information on sample composition and the species present in the sample is obtained from size distribution functions c(s).
(2) While not used much these days, in sedimentation equilibrium experiments at lower rotor speeds, diffusion opposes the process of sedimentation. When the two opposing forces reach equilibrium, the sample distribution curve is exponential in appearance, and molecular weights are obtained from this. Equilibria can be studied to yield dissociation constant Kd values.
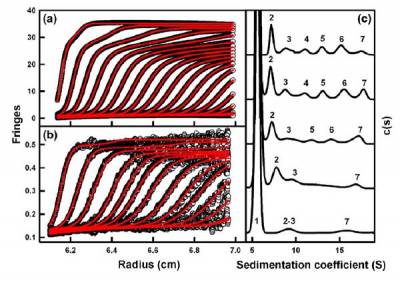
Factor H Oligomers by Sedimentation Velocity
In complement Factor H with 20 SCR domains, if there are at least two different self-dimerisation sites at SCR-6/8 and SCR-16/20, this leads to the prediction that the two dimer sites would lead to the formation of indefinite Factor H oligomers through the daisy-chaining of these two dimer sites. The presence of Factor H monomers, dimers and smaller amounts of larger species ranging up to heptamers were confirmed by size-distribution analyses c(s) of ultracentrifugation sedimentation velocity experiments. A series of peaks 2-7 in the c(s) plot corresponding to dimers to heptamers were directly seen as the Factor H concentration increased.
Publications:
Nan, R., Gor, J. & Perkins, S.J. (2008). Implications of the progressive self-association of wild-type human Factor H for complement regulation and disease. J. Mol. Biol. 375, 891-900
Okemefuna, A.I., Nan, R., Gor, J. & Perkins, S.J. (2009). Electrostatic interactions contribute to the folded-back conformation of wild-type human Factor H. J.Mol.Biol. 391, 98-118
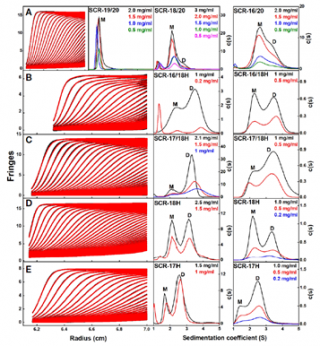
Location of a Factor H dimer site by AUC Sedimentation Velocity
One of the two dimer sites in Factor H was previously located to SCR-6/8. The location of the other one was somewhere in the SCR-16/20 region. Recombinant fragments of SCR-16/20 were used to pin down the second dimer site. The figure shows sedimentation velocity AUC runs for seven recombinant fragments of SCR-16/20. That for SCR-19/20 showed a monomer peak (M) only. Those involving SCR-17 and SCR-18 showed dimers peaks (D). By this the second dimer site was located.
Publication:
Dunne, O. M., Gao, X., Nan, R., Gor, J., Adamson, P. J., Gordon, D. L., Moulin, M., Haertlein, M., Forsyth, V. T. & Perkins, S. J. (2021) A dimerization site at SCR-17/18 in Factor H clarifies a new mechanism for complement regulatory control. Frontiers Immunol. 11, 601895.
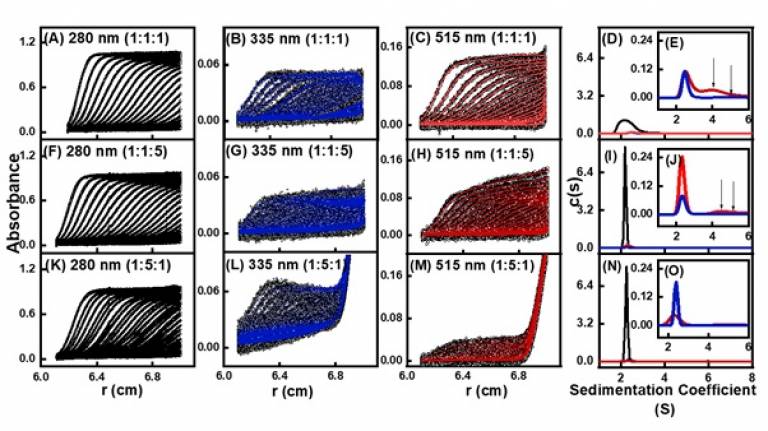
Multiwavelength AUC on the new Optima Instrument
Zinc a2 glycoprotein (ZAG) is a multifunctional glycoprotein with a class I MHC-like protein fold and an a1- a2 lipid-binding groove. It binds fatty acids in its groove. The physiological ZAG ligand is unknown, however this information is essential to understand ZAG function and its role in disease. Dr McDermott performed AUC titration studies with the fatty acids DAUDA and BODIPY. Each fatty acid binds to ZAG. The AUC analyses of different molar ratios of ZAG : DAUDA : BODIPY complexes at three different wavelengths showed that ZAG contains coincident binding sites for DAUDA and C16-BODIPY in which the ligands compete with each other in ternary complexes of both lipids in different molar ratios (see panels G,H and L,M). The more of one ligand that is present, the less is bound of the other ligand.
Publication:
Zahid, H., Lau, A. M., Kelly, S. M., Karu, K., Gor, J., Perkins, S. J. & McDermott, L. C. (2021) Identification of diverse lipid-binding modes in the groove of zinc-α2-glycoprotein reveals its functional versatility. FEBS J. 16293, 1-21.


 Close
Close