Decoding enzyme motions: Discovery of key relationships in enzymatic function
12 October 2023
In a new study published in Proceedings of the National Academy of Sciences (PNAS), the team has demonstrated, for the first time, the relationship between an enzyme’s motions and its catalytic rates and drug-binding capacities.
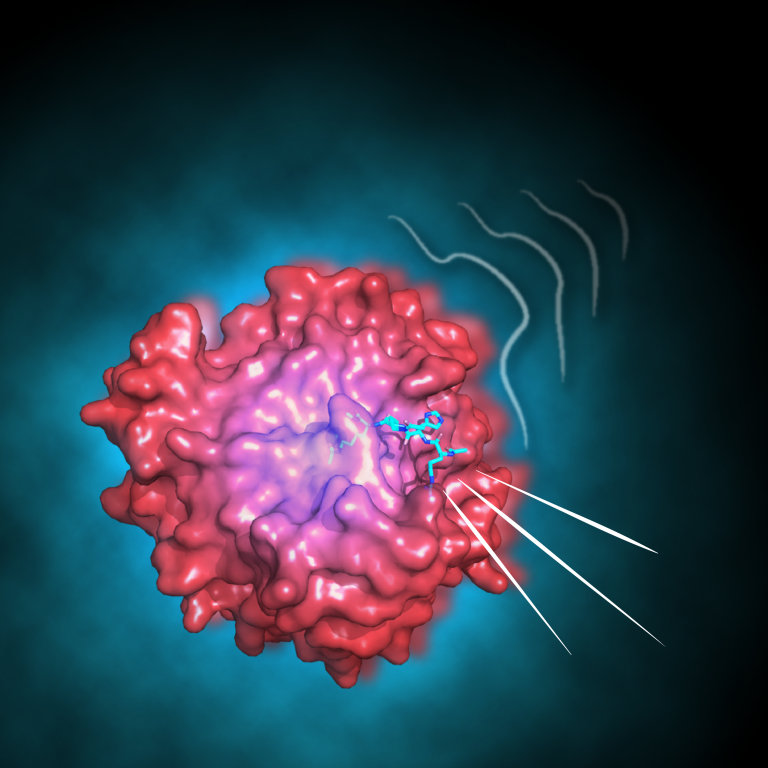
Professor Flemming Hansen and Dr Vaibhav Kumar Shukla have achieved a significant advancement in comprehending enzymatic function.
Enzymes are known to sample varied conformations on a millisecond timescale, which are often crucial for their biological function. The study focused on a specific enzyme, the histone deacetylase enzyme (HDAC8), and its mutants. The team shows four global kinetic parameters and a model of the intrinsic dynamics accurately describe the enzymatic activity, inhibitor affinity, and inhibitor residence-time.
Moreover, the study points out that the nuances in enzymatic activity and inhibitor potency can be discerned by observing the differences in their intrinsic dynamics. The impact of this research is profound, not only advancing our understanding of enzymatic regulations and the influence of missense mutants, but also potentially setting the stage for significant advancements in drug development and the logical design of enzymes.
With enzymes playing a central role in many drug discovery programmes, these findings have the potential to revolutionize and enhance drug development processes and methodologies related to enzyme designs.
Further Information:
Professor Flemming Hansen Staff Profile
 Close
Close

