Peter Klöhn
Research Synopsis
Prions, the infectious agents that cause the lethal brain disease Creutzfeldt-Jakob disease (CJD) in humans infect a range of different cells in the brain and in the periphery. In contrast to other infectious diseases, like viral or bacterial diseases, prions are not recognised as rogue proteins by the immune system and therefore expand rapidly and unhindered.
We seek to better understand how prions replicate in neuronal cells to identify novel ways for therapeutic intervention. Engineering of prion-susceptible cell models enables us to scrutinise the molecular underpinnings of protein misfolding. This helps us to identify gene regulatory networks that are associated with prion replication and secretion. Identification of antibodies that specifically recognise disease-associated forms of the prion protein in our laboratory will greatly support our endeavour to investigate prion replication in vivo and in vitro
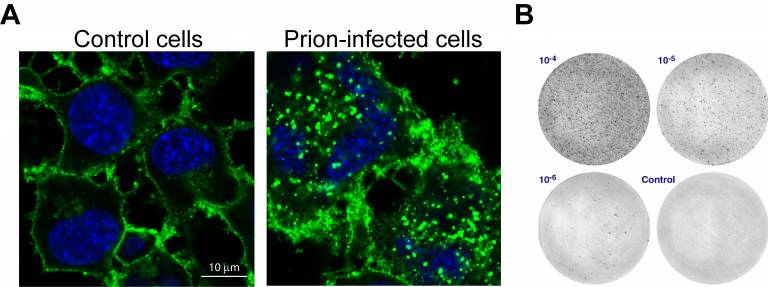 |
To investigate what gene-encoded proteins contribute to prion propagation we establish cell models and diagnostic assays. The prion protein (PrP) can be visualised in healthy and prion-infected cells with antisera against PrP (panel A). While PrP decorates the membranes in control cells, aggregates of misfolded PrP are apparent in the periphery of infected cells. The Scrapie Cell Assay helps us to quantify the degree of prion infection (panel B). |
Prion protein conversion at two distinct cellular sites precedes fibrillisation.
Ribes JM, Patel MP, Halim HA, Berretta A, Tooze SA, Klöhn PC.Nat Commun. 2023 Dec 15;14(1):8354. doi: 10.1038/s41467-023-43961-1.PMID: 38102121 Free PMC article.
Prion Propagation is Dependent on Key Amino Acids in Charge Cluster 2 within the Prion Protein.
Bhamra S, Arora P, Manka SW, Schmidt C, Brown C, Rayner MLD, Klöhn PC, Clarke AR, Collinge J, Jat PS.J Mol Biol. 2023 Feb 28;435(4):167925. doi: 10.1016/j.jmb.2022.167925. Epub 2022 Dec 16.PMID: 36535427
Philiastides A , Ribes JM , Yip DC , Schmidt C , Benilova I , Klöhn PC
Viruses. 2019 Sep 22;11(10). pii: E888. doi: 10.3390/v11100888.
Physical, chemical and kinetic factors affecting prion infectivity.
Properzi F, Badhan A, Klier S, Schmidt C, Klöhn PC, Wadsworth JD, Clarke AR, Jackson GS, Collinge J.
Prion. 2016 May 3;10(3):251-61. doi: 10.1080/19336896.2016.1181250.
A systematic investigation of production of synthetic prions from recombinant prion protein.
Schmidt C, Fizet J, Properzi F, Batchelor M, Sandberg MK, Edgeworth JA, Afran L, Ho S, Badhan A, Klier S, Linehan JM, Brandner S, Hosszu LL, Tattum MH, Jat P, Clarke AR, Klöhn PC, Wadsworth JD, Jackson GS, Collinge J.
Open Biol. 2015 Dec;5(12):150165. doi: 10.1098/rsob.150165.
Identification of a gene regulatory network associated with prion replication.
Marbiah MM, Harvey A, West BT, Louzolo A, Banerjee P, Alden J, Grigoriadis A, Hummerich H, Kan HM, Cai Y, Bloom GS, Jat P, Collinge J, Klöhn PC.
EMBO J. 2014 Jul 17;33(14):1527-47. doi: 10.15252/embj.201387150. Epub 2014 May 19.
Plasmacytoid dendritic cells sequester high prion titres at early stages of prion infection.
Castro-Seoane R, Hummerich H, Sweeting T, Tattum MH, Linehan JM, Fernandez de Marco M, Brandner S, Collinge J, Klöhn PC.
PLoS Pathog. 2012 Feb;8(2):e1002538. doi: 10.1371/journal.ppat.1002538. Epub 2012 Feb 16.
PrP antibodies do not trigger mouse hippocampal neuron apoptosis.
Klöhn PC, Farmer M, Linehan JM, O'Malley C, Fernandez de Marco M, Taylor W, Farrow M, Khalili-Shirazi A, Brandner S, Collinge J.
Science. 2012 Jan 6;335(6064):52. doi: 10.1126/science.1215579.
 Close
Close


