Alzheimer’s disease acquired from historic medical treatments
30 January 2024
Five cases of Alzheimer’s disease are believed to have arisen as a result of medical treatments decades earlier, reports a team of UCL and UCLH researchers.
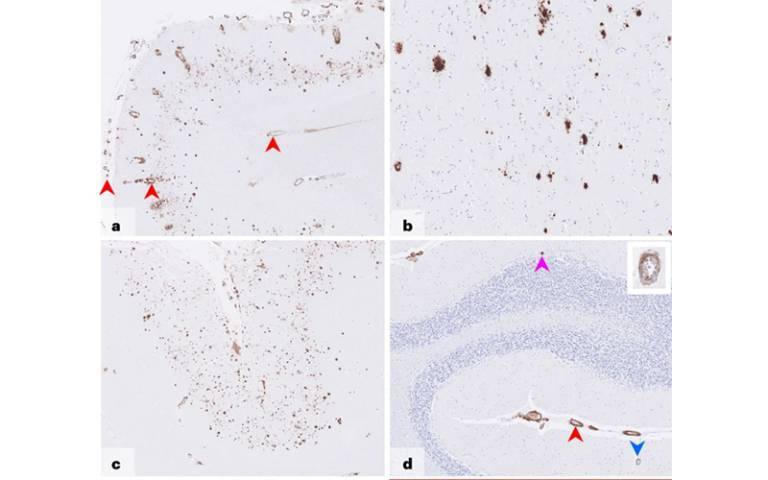
Alzheimer’s disease is caused by the amyloid-beta protein, and is usually a sporadic condition of late adult life, or more rarely an inherited condition that occurs due to a faulty gene. The new Nature Medicine paper provides the first evidence of Alzheimer’s disease in living people that appears to have been medically acquired and due to transmission of the amyloid-beta protein.
The people described in the paper had all been treated as children with a type of human growth hormone extracted from pituitary glands from deceased individuals (cadaver-derived human growth hormone or c-hGH). This was used to treat at least 1,848 people in the UK between 1959 and 1985, and used for various causes of short stature. It was withdrawn in 1985 after it was recognised that some c-hGH batches were contaminated with prions (infectious proteins) which had caused Creutzfeldt-Jakob disease (CJD) in some people. c-hGH was then replaced with synthetic growth hormone that did not carry the risk of transmitting CJD.
These researchers previously reported that some patients with CJD due to c-hGH treatment (called iatrogenic CJD) also had prematurely developed deposits of the amyloid-beta protein in their brains.* The scientists went on to show in a 2018 paper that archived samples of c-hGH were contaminated with amyloid-beta protein and, despite having been stored for decades, transmitted amyloid-beta pathology to laboratory mice when it was injected.** They suggested that individuals exposed to contaminated c-hGH, who did not succumb to CJD and lived longer, might eventually develop Alzheimer’s disease.
This latest paper reports on eight people referred to UCLH’s National Prion Clinic at the National Hospital for Neurology and Neurosurgery in London, who had all been treated with c-hGH in childhood, often over several years.
Five of these people had symptoms of dementia, and either had already been diagnosed with Alzheimer’s disease or would otherwise meet the diagnostic criteria for this condition; another person met criteria for mild cognitive impairment. These people were between 38 and 55 years old when they started having neurological symptoms. Biomarker analyses supported the diagnoses of Alzheimer’s disease in two patients with the diagnosis, and was suggestive of Alzheimer’s in one other person; an autopsy analysis showed Alzheimer’s pathology in another patient.
The unusually young age at which these patients developed symptoms suggests they did not have the usual sporadic Alzheimer’s which is associated with old age. In the five patients in whom samples were available for genetic testing, the team ruled out inherited Alzheimer’s disease.
As c-hGH treatment is no longer used, there is no risk of any new transmission via this route. There have been no reported cases of Alzheimer’s acquired from any other medical or surgical procedures. There is no suggestion that amyloid-beta can be passed on in day-to-day life or during routine medical or social care.
However, the researchers caution that their findings highlight the importance of reviewing measures to ensure there is no risk of accidental transmission of amyloid-beta via other medical or surgical procedures which have been implicated in accidental transmission of CJD.
The lead author of the research, Professor John Collinge, Director of the UCL Institute of Prion Diseases and a consultant neurologist at UCLH, said: “There is no suggestion whatsoever that Alzheimer’s disease can be transmitted between individuals during activities of daily life or routine medical care. The patients we have described were given a specific and long-discontinued medical treatment which involved injecting patients with material now known to have been contaminated with disease-related proteins.
“However, the recognition of transmission of amyloid-beta pathology in these rare situations should lead us to review measures to prevent accidental transmission via other medical or surgical procedures, in order to prevent such cases occurring in future.
“Importantly, our findings also suggest that Alzheimer's and some other neurological conditions share similar disease processes to CJD, and this may have important implications for understanding and treating Alzheimer’s disease in the future.”
Co-author Professor Jonathan Schott (UCL Queen Square Institute of Neurology, honorary consultant neurologist at UCLH, and Chief Medical Officer at Alzheimer’s Research UK) said: “It is important to stress that the circumstances through which we believe these individuals tragically developed Alzheimer’s are highly unusual, and to reinforce that there is no risk that the disease can be spread between individuals or in routine medical care. These findings do, however, provide potentially valuable insights into disease mechanisms, and pave the way for further research which we hope will further our understanding of the causes of more typical, late onset Alzheimer’s disease.”
First author Dr Gargi Banerjee (UCL Institute of Prion Diseases) said: “We have found that it is possible for amyloid-beta pathology to be transmitted and contribute to the development of Alzheimer’s disease. This transmission occurred following treatment with a now obsolete form of growth hormone, and involved repeated treatments with contaminated material, often over several years. There is no indication that Alzheimer’s disease can be acquired from close contact, or during the provision of routine care.”
The study was supported by the Medical Research Council, the National Institute for Health and Care Research (NIHR), the NIHR UCLH Biomedical Research Centre, Alzheimer’s Research UK, and the Stroke Association.
If you were treated with the growth hormone (c-hGH) in the UK between 1959 and 1985 and would like further information about this research, please contact the National Prion Clinic via email (uclh.prion.help@nhs.net) or by telephone (020 7679 5142 or 020 7679 5036).
 Close
Close

