New IPLS paper in Nature Physics by Dr Andela Saric: Self-replication of Protein Fibrils
18 July 2016
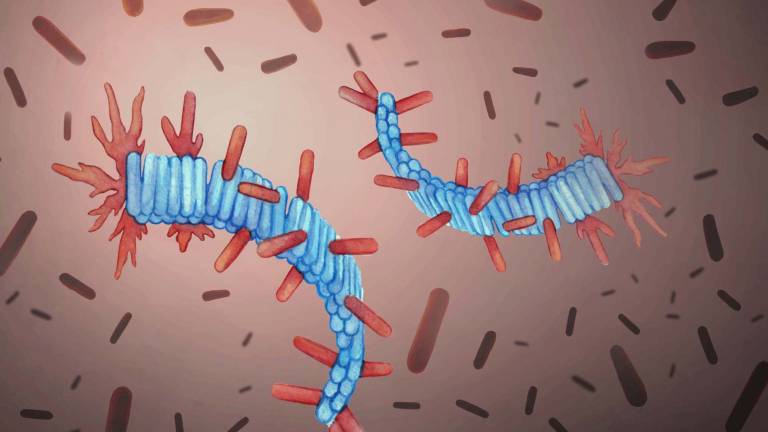
The ability of biological molecules to replicate themselves is the foundation of life, and it usually involves a complex cellular machinery. However, certain protein structures promote their own replication, without any additional assistance. One example is the self-replication of pathological protein fibrils, called amyloids, involved in neurodegenerative disorders, including Alzheimer's and Parkinson's. It is these fibrils, when intertwined and entangled with each other, that for instance form plaques in the brains of Alzheimer's patients.
Spontaneous formation of the first amyloid fibrils is very slow, and it typically takes several decades. But once the first fibrils are formed, they catalyse their own replication. This process leads to the fast proliferation of pathological species involved in the diseases, making it extremely challenging to control. Despite its importance, the fundamental mechanism of how protein fibrils can self-replicate, without any additional machinery, is not well understood.
Artist's rendering of protein fibrils (in blue) and healthy proteins (in red) from computer simulations. Credit: Ivan Barun, dr. med.
In the recent paper published in Nature Physics, the IPLS Fellow A. Saric and colleagues use a powerful combination of computer simulations and biophysical experiments to identify the necessary requirements for the self-replication of protein fibrils. Their main finding is that the seemingly complicated process of the fibril self-replication is governed by a simple physical mechanism: deposition of healthy proteins onto the surface of existing fibrils. Taking Alzheimer's Aβ peptide as a model system, they show a quantitative connection between the amount of healthy proteins that are deposited onto the existing fibrils, and the rate of the fibril self-replication. As a proof of principle, they further demonstrate that by modulating the interaction of healthy proteins with the surface of fibrils, they can control the fibril self-replication.
The results of Saric et al. suggest that controlling the deposition of monomeric proteins onto the surface of the existing protein fibrils may represent a fruitful strategy for limiting the spreading of pathological protein aggregates in a disease context. Moreover, these findings may be of great interest for nanotechnology, where achieving efficient self-replication in manufacturing of nano-materials is one of the unfulfilled goals.
 Close
Close

