Niral Patela,b, Adam Badarb, Mark Lythgoeb, Erik Årstada
a Institute of Nuclear Imaging, University College Hospital, 235 Euston Road, London NW1 2BU, UK; b
Centre for Advanced Biomedical Imaging, Division of Nuclear Medicine
and Institute of Child Health, University College London, 72 Huntley
Street, London, WC1E 6DD, UK.
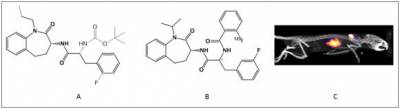
Voltage Gated Sodium Channels (VGSCs) are integral membrane proteins, initiating and propagating action potentials in neurones and other electrically excitable cells.1 They therefore allow co-ordination and communication between neurons, particularly when speed is of the essence. Dysfunction and/or overexpression of VGSCs have been associated with a number of pathological conditions including multiple sclerosis, epilepsy and cancer. Therefore, there is increasing rationale for imaging VGSC in order to provide earlier diagnosis and aid in more effective treatments. Despite its great potential, the imaging of VGSC remains largely unexplored. Recently, the tritiated benzazepinone derivative has been shown to block the VGSC isoform NaV1.7 with high potency (Kd = 1.53 ± 0.46 nM, Bmax = 3.4 ± 1.2 pmol/mg of protein).2 In order to assess the suitability of benzazepinones as radiotracers for imaging VGSCs, the [I125]-labelled diastereomers of the BNZA analogue has been prepared and characterised by members within our group. Whilst one of the diastereomers demonstrated excellent metabolic stability, it suffered from poor brain penetration. A reason for this is due to its high lipophilicity, (log D7.4 of 3.93 ± 0.01), resulting in low specific binding and high plasma protein binding in vivo. Our current goal is to synthesise novel BNZA derivatives with lower lipophilicity. These tracers will then be tested in vitro and by in vivo SPECT and PET imaging in order to determine if VGSCs have been successfully targeted.
References:
1 Catterall, W. A. From
ionic currents to molecular mechanisms: the structure and function of
voltage-gated sodium channels. Neuron 2000, 26 (1), 13-25;
2
Williams, B. S.; Felix, J. P.; Priest, B. T.; Brochu, R. M.; Dai, K.;
Hoyt, S. B.; London, C.; Tang, Y. S.; Duffy, J. L.; Parsons, W. H.;
Kaczorowski, G. J.; Garcia, M. L. Characterization of a new class of
potent inhibitors of the voltage-gated sodium channel Nav1.7.
Biochemistry 2007, 46 (50), 14693-14703.
 Close
Close

