Mapping the evolution of E. Coli virulence offers refined drug target
15 June 2023
A multi-centre team led by UCL, the Wellcome Sanger Institute, the University of Oslo and Imperial College London have shown how targeting the E. coli bacterium’s protective capsule could help to prevent and treat bloodstream infections.
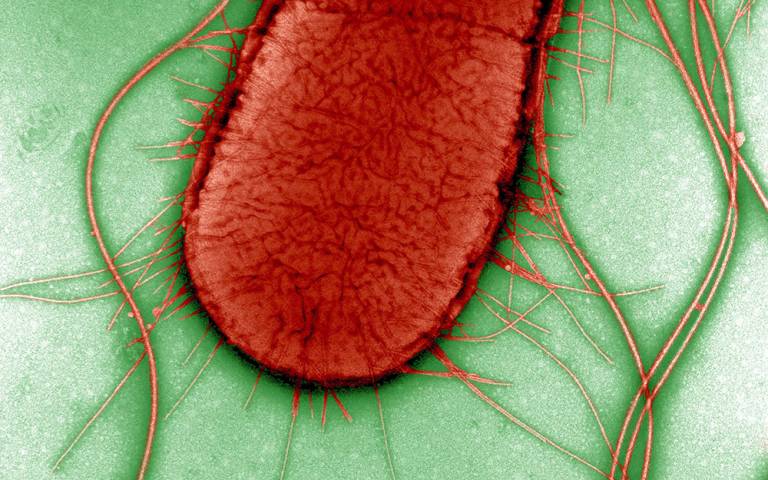
The new study, published in Nature Communications, is the first to map the evolutionary timeline and population distribution of Escherichia coli’s protective outer capsule, which is responsible for the bacterium’s virulence. The study also shows how targeting the bacterium’s protective layer can help treat extraintestinal infections.
This new work focused on a particular subset of E. coli with a specific capsule – the extracellular barrier that surrounds a bacterium – which scientists have called K1. E. coli. This type of capsule is known to cause invasive diseases such as bloodstream or kidney infections, and meningitis in newborns, because it allows the bacterium to mimic molecules already present in human tissues and enter the body unnoticed.
The researchers present evidence that targeting the capsule can be used as the basis of treatment, paving the way to prevent serious E. coli infections.
E. coli is a common cause of urinary tract and bloodstream infections and can cause meningitis in premature and term newborns, with a mortality rate as high as 40 per cent. Furthermore, the rise in hypervirulent and multi-drug resistant E. coli during the last decade means that developing effective strategies to prevent and treat E. coli has now become urgent. Understanding the bacterium’s anatomy and how this plays a role in causing disease is key for the prevention of serious infections. Scientists until now lacked basic knowledge of the prevalence, evolution and functional properties of the K1 capsule, limiting their capacity to combat E. coli infections.
In the new study, the research team mapped the evolution, prevalence and distribution of this E. coli strain. Using high-resolution population genomics, whole genome sequencing and advanced computational tools, they analysed 5,065 clinical samples from different countries and time periods. The data included large collections of samples from the UK and Norway, newly-generated adult and neonatal samples from six countries, such as Brazil, Mexico and Laos among others, and samples from the pre-antibiotic era from 1932 onwards.
They found that this specifically virulent capsule K1 actually dates further back in time, approximately 500 years earlier than previously imagined. This highlights the importance of the capsule for the bacterium’s survival and the role of the extracellular barrier in the success of E. coli as the main cause of extraintestinal infections.
Dr Sergio Arredondo-Alonso, lead author of the study from the University of Oslo and the Wellcome Sanger Institute, said: “It was exciting to discover the possibility of reconstructing the evolutionary history of the K1 capsule over the last half millennium, and to see how the capsule genes have been acquired over and over again by many different lineages of this pathogen species over the centuries. As neither the prevalence nor the history of K1 was known, it felt like we entered truly unchartered territory and significantly advanced understanding of this major pathogen species.”
The study also shows that 25 per cent of all current E. coli strains responsible for blood infections contain the genetic information needed to develop the K1 capsule. Obtaining a complete evolutionary history of this strain will now allow researchers to understand how bacteria obtain the genetic material responsible for severe virulence in the first place, and analyse ways to combat them.
Emeritus Professor Peter Taylor (UCL School of Pharmacy), a co-senior author of the study, said: “Although E. coli is commonly found in a variety of extraintestinal infections, it doesn’t have a substantial array of virulence factors that might account for its invasive and persistence characteristics. Rather, in many infectious scenarios it enters the body and persists through stealth. It has been evident from the 1960s onwards that a large majority of neonatal systemic infections, such as meningitis and septicaemia, are caused by E. coli colonisers that express the K1 antigen. This mimetic of host antigens enables the pathogen to avoid the immature immune defences of the newborn host. We now determine that the K1 polymer is also a main feature of adult systemic E. coli infections. We have previously shown that enzymatic removal of the K1 capsule can prevent and even cure potentially lethal experimental infections, making this a promising paradigm for the treatment of antibiotic-resistant persisters.”
By using enzymes from bacteriophages, which are viruses that ‘infect and kill’ bacteria, researchers were able to remove the bacterium’s extracellular barrier and make it vulnerable to the human immune system. The researchers demonstrated in in vitro studies using human serum that targeting this capsule can be a way to broadly treat E. coli infection without the use of antibiotics, consistent with previous experimental infections in animals.
Professor Jukka Corander, a co-senior author of the study from the Wellcome Sanger Institute and the University of Oslo, said: “Our research shows the importance of representative genomic surveys of pathogens over time and space. These studies will enable us to reconstruct the evolutionary history of successful bacterial lineages and pinpoint changes in their genetic make-up that can lead to their ability to spread and cause disease. Such knowledge is ultimately providing the basis for designing future interventions and therapies against these pathogens.”
Links
- Emeritus Professor Peter Taylor's academic profile
- UCL School of Pharmacy
- UCL Faculty of Life Sciences
Source
Image
Credit: David Gregory and Debbie Marshall, Wellcome Images, CC-BY-4.0
Media contact
Dr Matt Midgley
E: m.midgley [at] ucl.ac.uk
 Close
Close

