CardioQ: Improved surgical outcomes through perioperative circulatory optimisation
12 December 2014
Professor Mervyn Singer designed and developed a new device - the CardioQ Oesophageal Doppler Monitor - for use in monitoring critically ill patients, and those undergoing high-risk surgery. Over half a million patients have now benefitted from this technology, which reduces postoperative complication rates and length of time in hospital. The device is now recommended in National Institute for Health and Clinical Excellence guidance and has been identified by the Department of Health as one of six high-impact innovations to be implemented fully across the NHS.
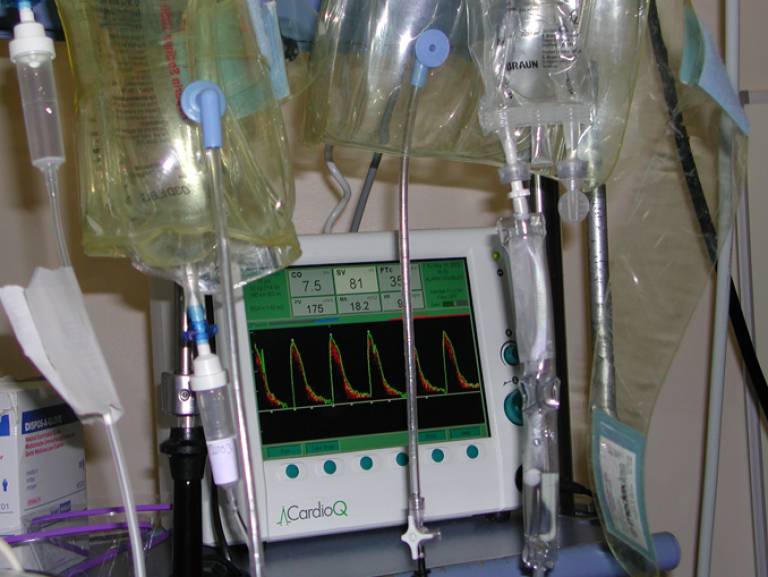
The CardioQ Oesophageal Doppler Monitor was developed by Professor Singer (UCL Medicine) to monitor blood flow in critically ill patients, and those undergoing high-risk surgery. It uses a probe inserted via the mouth into the oesophagus. The probe is connected to a monitor that displays flow velocity waveforms of blood being pumped down the descending thoracic aorta. This information can be used to quickly detect any deterioration in circulatory status, and to guide optimal fluid and drug therapy.
Between 1993 and 2004, Professor Singer conducted a series of trials to assess the device in patients undergoing cardiac surgery, or repair of fractured hips. The trials reported significant reductions in postoperative complications and hospital stay compared to standard care. The device is now produced and sold commercially by Deltex Medical, a British company based in Chichester.
The CardioQ has changed the way in which doctors can care for patients having major surgery or in intensive care. It allows doctors to intervene quickly and safely based on small changes in circulating blood volume and so avoid the dangers of reduced oxygen delivery.
To date, more than half a million patients have benefitted from the use of the CardioQ in surgery and in intensive care. By the end of 2012, a total of 926 monitors had been installed in the UK and monitors have been sold widely in many other countries including the US, Canada, South America and Continental Europe.
The benefits of using the device (reduced complications, shorter ICU and hospital stay) have been evaluated and endorsed independently by the US Agency for Healthcare Research and Quality and the NIHR Health Technology Assessment Programme. In March 2011 NICE published its Medical Technologies Guidance (MTG3) recommending the use of oesophageal Doppler monitoring (ODM) in high-risk surgery. The National Institute for Health and Clinical Excellence estimated that its use could save around £1,000 each time it is used for high-risk surgery, and up to £400m per year for the NHS as a whole.
In the same year, the NHS Innovative Technology Adoption Procurement Programme selected oesophageal Doppler-guided intra-operative fluid management as one of three technologies for wider adoption by the NHS in England. Later that year, the NHS Innovation Health & Wealth Review named ODM as one of six high-impact innovations and called for the widespread implementation of ODM for fluid management in surgery, stating that this technology "can reduce mortality rates for elective procedures, improve the quality of care for more than 800,000 patients a year, and save the NHS at least £400m annually".
In May 2012, the NHS National Technology Adoption Centre published its Intraoperative Fluid Management Technologies (IOFMT) Adoption Pack to encourage adoption throughout the NHS as a recommended High Impact Innovation. Hospital trusts have to implement ODM at projected target levels in 2013/14 or lose access to their CQUIN payments, which make up 2.5% of their total budget.
Looking outside the UK, the device has been adopted or is under formal evaluation by health regions and large hospital groups in the USA, France, Spain, and Canada. In 2013, the US Centres for Medicare and Medicaid Services (CMS) granted ODM its own unique code for physician reimbursement, while the professional body for anaesthetists in France, Société Française d'Anesthésie et de Réanimation, published new guidelines setting out recommended fluid management best practice for its members. These guidelines make it clear that ODM-guided fluid management should be used in all high-risk surgery in France, estimated to cover circa 750,000 patients a year.
Deltex Medical won the National Outstanding Achievement category in the 2013 UK Healthcare Business Awards held at the NHS Healthcare Innovation Expo.
Related links
Image
- The Doppler machine (Mervyn Singer)
 Close
Close

