Find out about Professor Ronjon Chakraverty's research on allogeneic haematopoietic stem cell transplantation.
Allogeneic haematopoietic stem cell transplantation (allo-HSCT) is the most common form of adoptive T cell therapy for cancer. At UCL, we perform about 300 HSCT procedures per year making us one of the largets programs worldwide (Image by Sanjiv Luther, University of Lausanne).
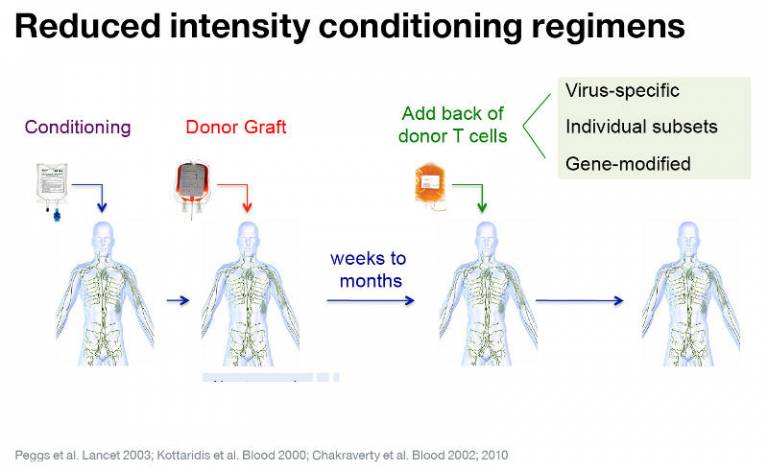
UCL pioneered the use of reduced intensity conditioning regimes that permit durable engraftment of donor followed by adoptive transfer of unmodified donor T cells to eradicate residual haematological cancers. More recently, our program has been testing strategies to modify the specificity or other properties of transferred T cells to improve their efficacy.
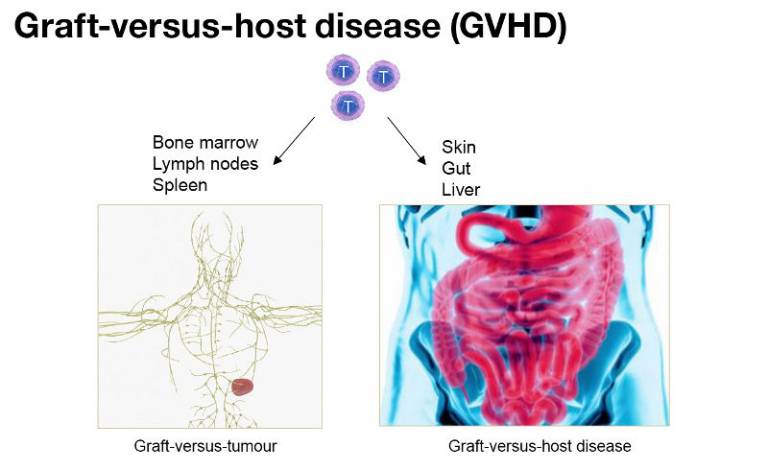
GVHD is a mjor clinical problem associated with allo-HCST. Following allo-HSCT, activated donor T cells are recruited to peripheral organs where they cause severe injury, e.g. colitis or dermatitis. A similar immune reaction can occur in the lymphoid organs and bone marrow, a process co-opted therapeutically to achieve a graft-versus-leukaemia (GVL) effect. A key challenge is to identify cellular and molecular mechanisms that distinguish GVL from GVHD.
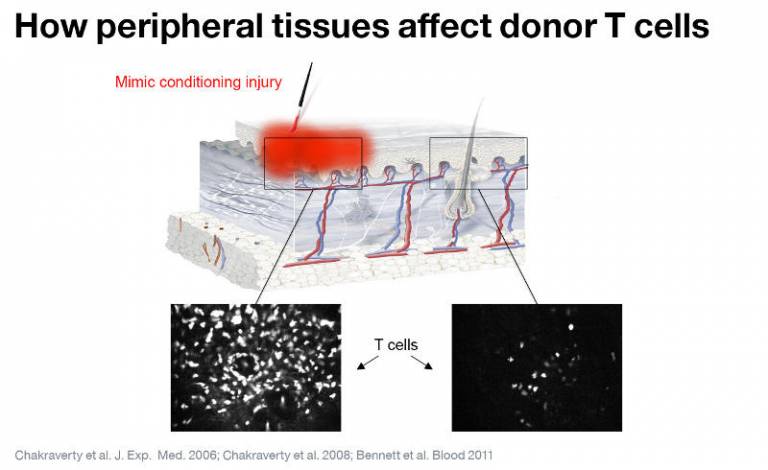
We are interested in the concept that peripheral tissues actively shape the functions of activated donor T cells. In the skin, we have shown that although allo-antigens are required for GVHD to occur, they are insufficient. Injury to host tissues that occurs following conditioning is required to induce immunopathology, as shown in this experiment where GVHD was induced only at the site of inflammation.

In our model systems, we have shown that Langerhans cells (specialised antigen-presenting cells present in the epidermis) are required for GVHD to occur in the skin but not elsewhere. Our current research is focussed upon understanding how donor T cells and Langerhans cells interact in the skin.
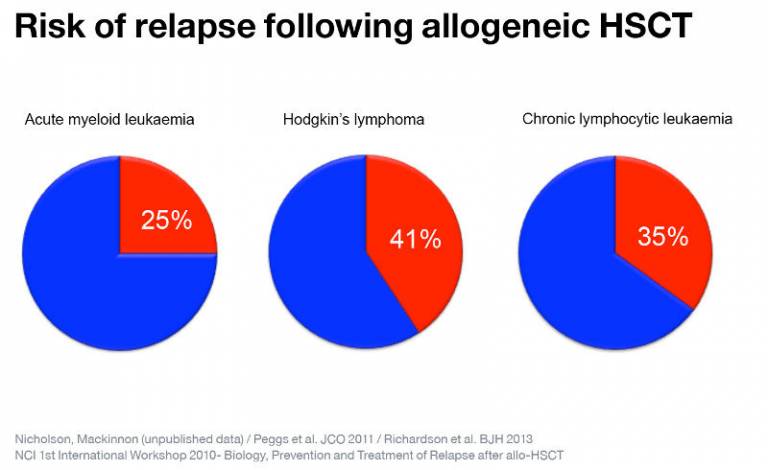
Although allo-HSCT is often effective in curing patients with haematological malignancies, the most common cause for treatment failure is relapse. Cancers may relapse as a result of immunoediting wherein tumour cells adapt to the selective immune pressure of the donor immune system and escape its control (e.g. through loss of antigens). However, we have shown that anti-tumour responses fail independently of tumour adaptation as a result of T cell 'exhaustion'.
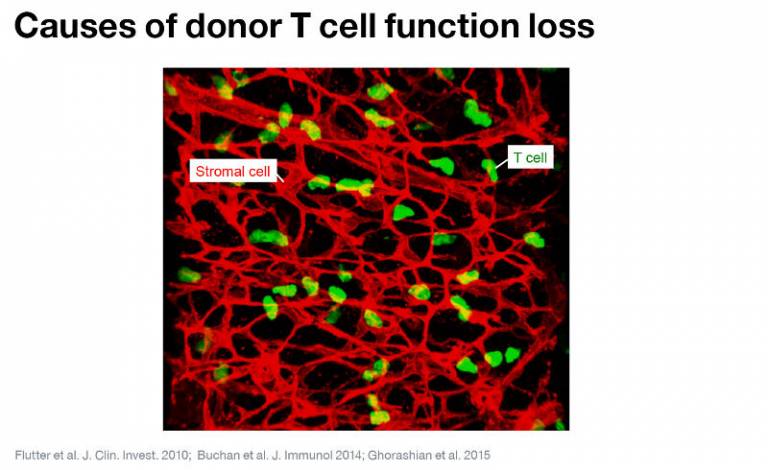
Donor T cells may recognise antigens on tumours that are also expressed on normal, non-bone marrow-derived cells (for exampl, upon stromal cells within secondary lymphoid organs). Under these circumstances, T cells gradually become 'exhausted' and lose key functions required to kill tumour cells.

We have shown that the functions of the exhausted donor T cells can be recovered by using antibodies that either block co-inhibitory receptors (e.g. PD-1) or that stimulate co-stimulatory receptors (e.g. OX40 or CD27). The challenge now is to design strategies that can be translated into the clinic.
 Close
Close

