Find out about the Institute's work on Scleroderma.
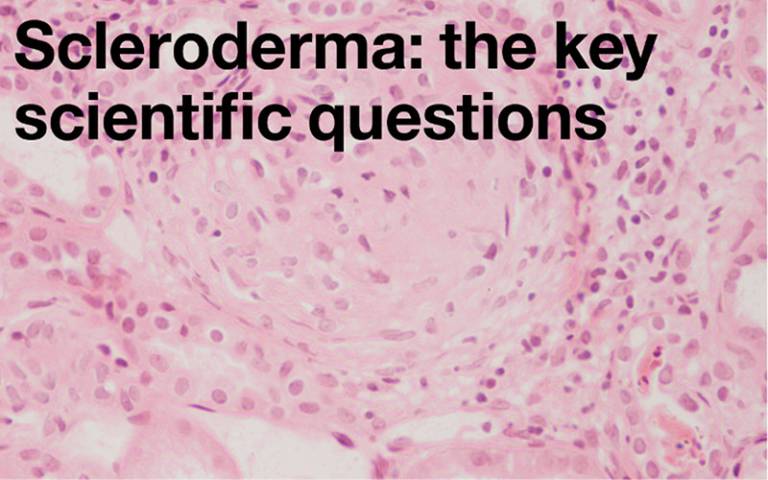
How does inflammation and autoimmunity trigger fibrosis? What prevents the process switching off and leading to scarring? Can factors that link inflammation, blood vessel damage and scarring be identified? Do these provide new targets for better treatment? What determines the pattern and severity of scleroderma when it develops?
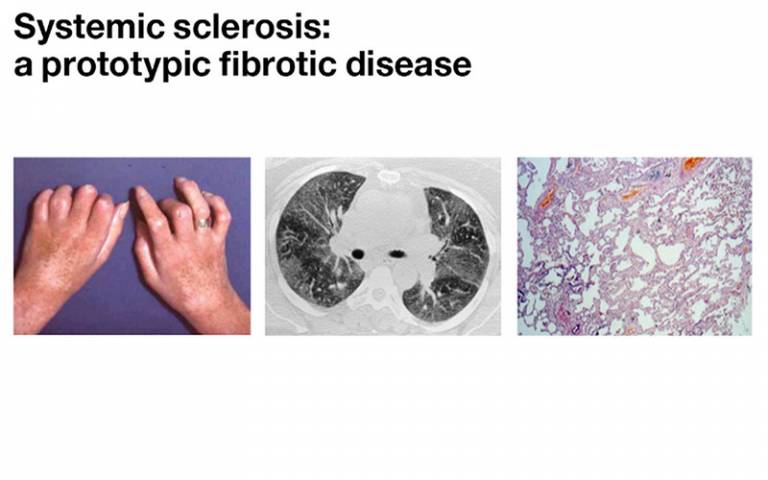
Prototypic fibrotic (PF) diseases are heterogeneous. Which patients get it? And which subset? If epith markers are predictive of decline (DPTA, KL6), in which gp most useful? What animal work can unravel the complex interaction between epithelial dysfunction and dysregulated ECM production by fibroblasts? To definitively evaluate the role of AEC dysfunction in SScPF, we need a translational approach.

A brief overview: Scleroderma clearly involves the blood vessels, immune system and scarring processes in the skin and other organs – but so do other diseases. Scleroderma is rare. It affects 1 in 10,000 in UK, 4F:1M, peak onset 30–60 years.
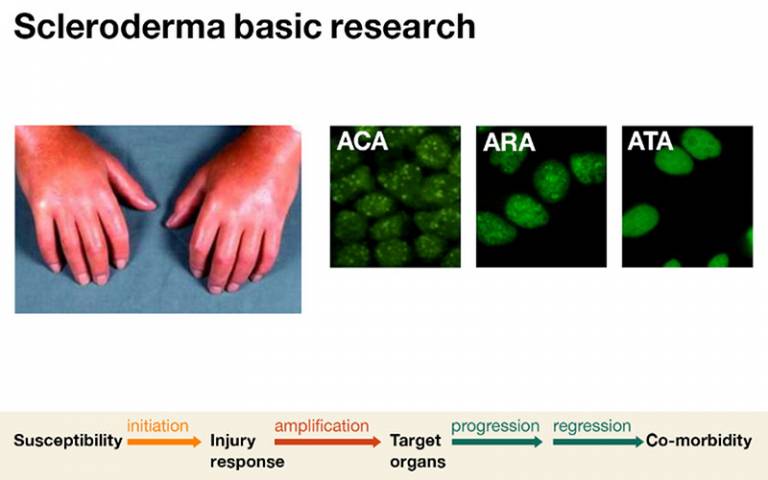
Our basic science research involves identifying the link between autoimmunity and failed tissue repair.
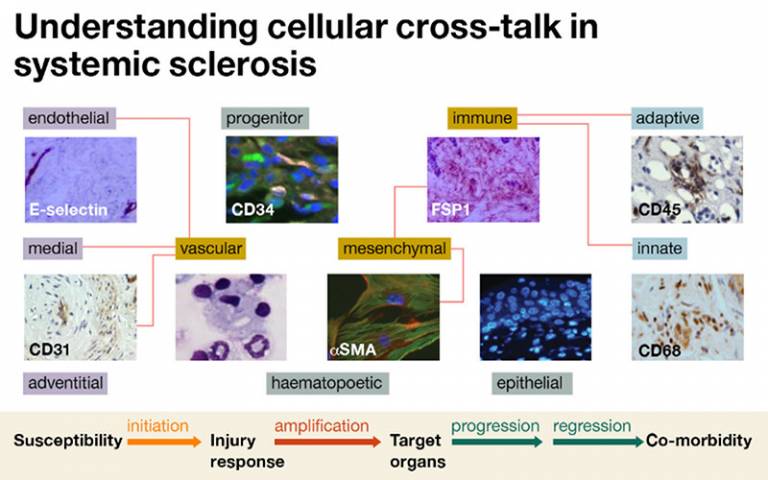
Multiple cell types are likely to be involved in systemic sclerosis, and the way that these communicate and interact is likely to be central to how the disease works.
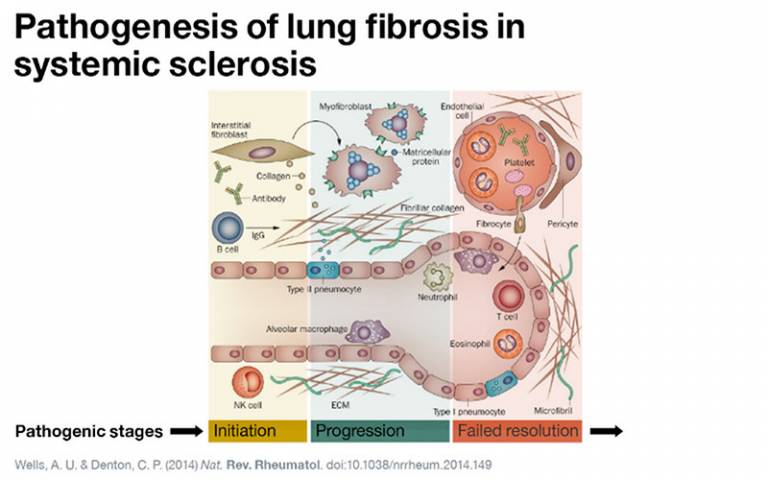
Initiation: Epithelial damage / Endothelial activation / Immune cell infiltration / Inflammation
Progression: Fibroblast proliferation / Fibrocyte recruitment / Epithelial-mesenchymal transition / Ongoing epithelial damage
Failed resolution: Myofibroblast persistence / Altered matricellular interaction / Perturbed epithelial repair / Ongoing epithelial damage

Experimental models allow genes and proteins that alter the degree of fibrosis or scarring after lung injury to be identified. This allows factors that may reduce fibrosis to be identified.
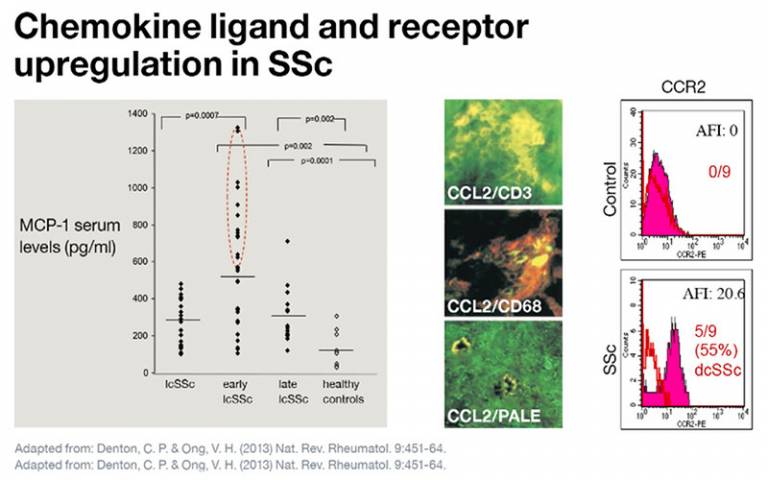
Chemokines such as MCP-1 (also called CCL2) have emerged as important factors that can link inflammation and fibrosis in systemic sclerosis. These are increased in blood of patients with lung fibrosis and their receptors (CCR2) expressed on fibroblasts in skin, suggesting that they can regulate cell trafficking and the location of fibrotic responses.
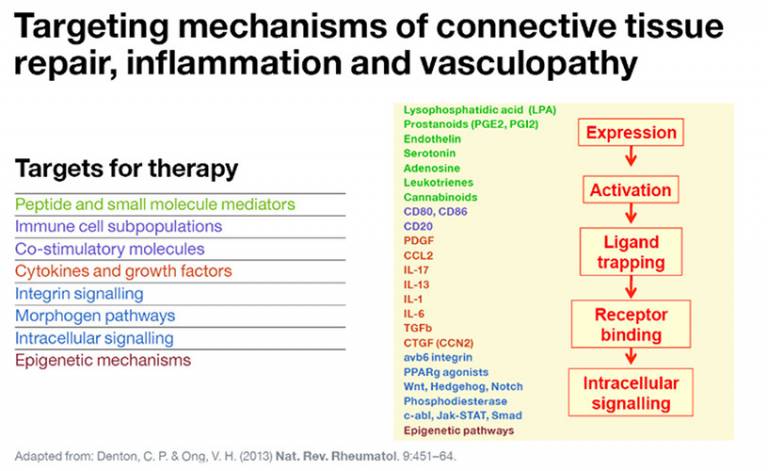
Results of systems biology studies enable key pathways to be identified that underpin communication between key cell types in systemic sclerosis. The components of these biological pathways may then become targets for new therapies.

Integrative approaches that link understanding of disease biology will allow targeting of factors that modulate multiple cell types and allow the driving processes of systemic sclerosis to be targeted. Rather than targeting one aspect of the disease, these approaches will benefit inflammation, autoimmunity, vascular damage and scarring within the diseases.
 Close
Close

