Find out about Professor Amit Nathwani's research on immunotherapy for chronic leukaemia.
Chronic lymphoid leukaemia (CLL), is the most common type of leukaemia in adults with 3,200 cases diagnosed each year in the UK. Leukaemia’s are cancers of the white blood cells (leukocytes). CLL affects B cell lymphocytes. B cells originate in the bone marrow, develop in the lymph nodes, and normally fight infection by producing antibodies.
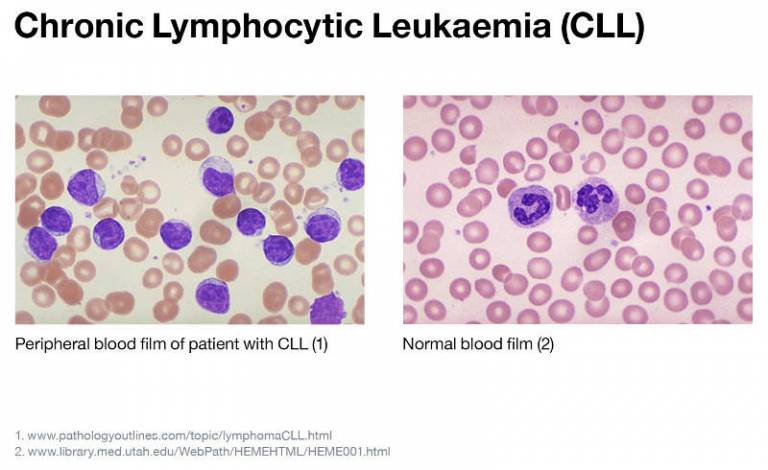
In CLL, B cells grow out of control and accumulate in the bone marrow, blood, and lymph nodes where they replace normal healthy blood cells. CLL remains a largely incurable disease. Therefore, our overriding aim is to develop immunotherapy therapy that involves engineering patients’ own immune cells to recognise and attack CLL cells.
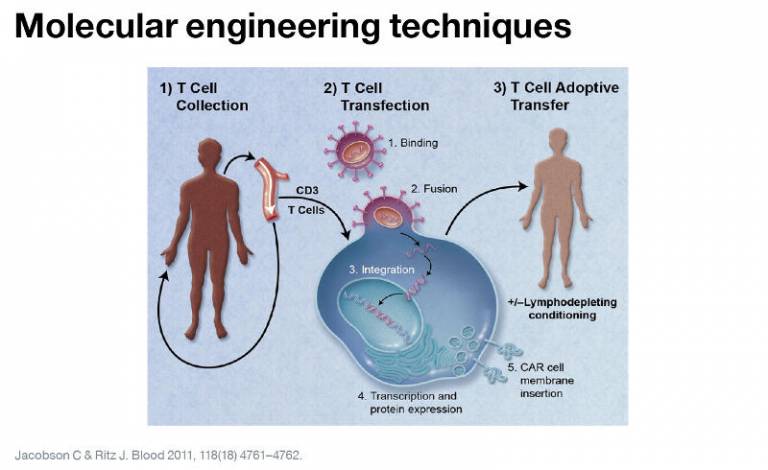
Our research is focussed on using molecular engineering techniques to modify the patients’ own immune cells (T-lymphocytes) to identify and kill the malignant CLL cells. To achieve this, the T-lymphocytes are isolated from the peripheral blood of the patient and transduced with a virus to express a Chimeric Antigen Receptor (CAR) specific for a protein (antigen) expressed by the malignant B-lymphocytes.
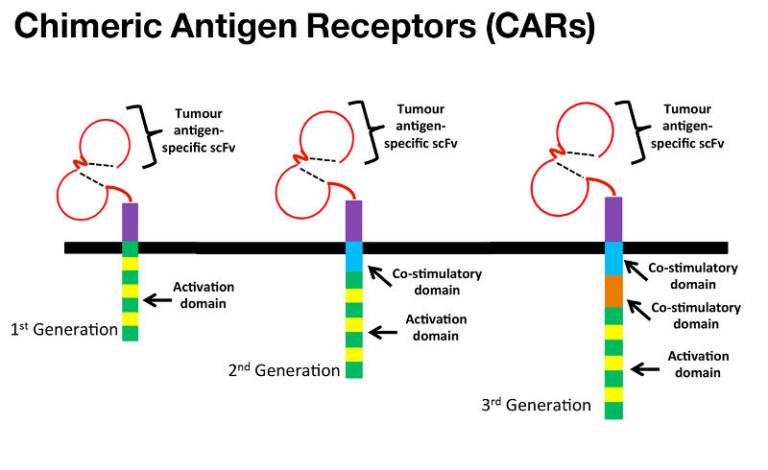
Binding of the CAR the antigen for which they are specific leads to T-lymphocyte activation and target cell death. The specificity of a CAR for a particular tumour antigen is tipically determined by the antigen binding regions of an antibody (scFv). The scFv is fused to either an activation domain alone (1st generation CAR), or along with one or two co-stimulatory domains (2nd and 3rd Chimeric Antigen Receptors (CARs) generation CAR respectively), resulting in T-cell activation upon target cell binding.
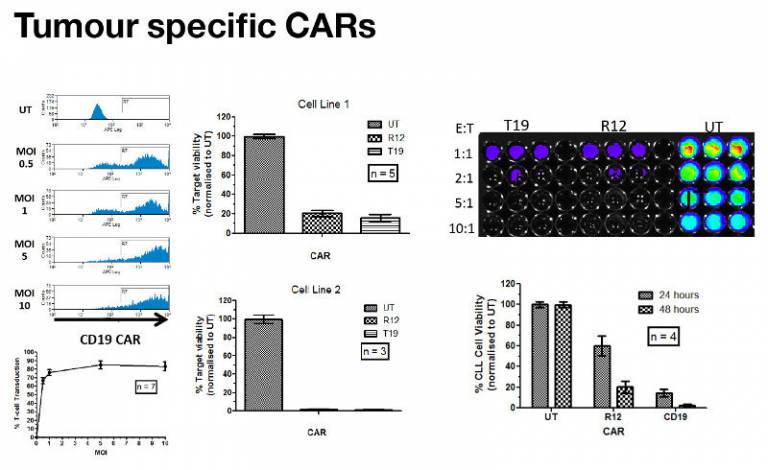
CARs targeting the ubiquitous B-lymphocyte target antigen CD19 have shown substantial promise in Phase I clinical trials in the USA. In addition to preparing for a CD19 CAR trial here in the UK, we have also been developing a CAR against an alternative antigen expressed by malignant B-cells such as ROR1. High levels of gene transfer into CLL patient T-lymphocytes has been achieved by our group. This results in significant killing of cell lines and CLL cells.
 Close
Close

