Find out about Professor Amit Nathwani's research on haemophilia gene therapy.
Haemophilia is an X linked bleeding diathesis. There are two types – A and B – both due to deficiencies in a plasma protein (Factor VIII and Factor IX respectively) which are key in the coagulation cascade.
Haemophilia is an ideal target for gene therapy as a small increase in the circulating level of FVIII or FIX from 5% of normal, will change the disease phenotype from severe with spontaneous bleeding, to a moderate form in which bleeding is only typically seen after injury/surgery.
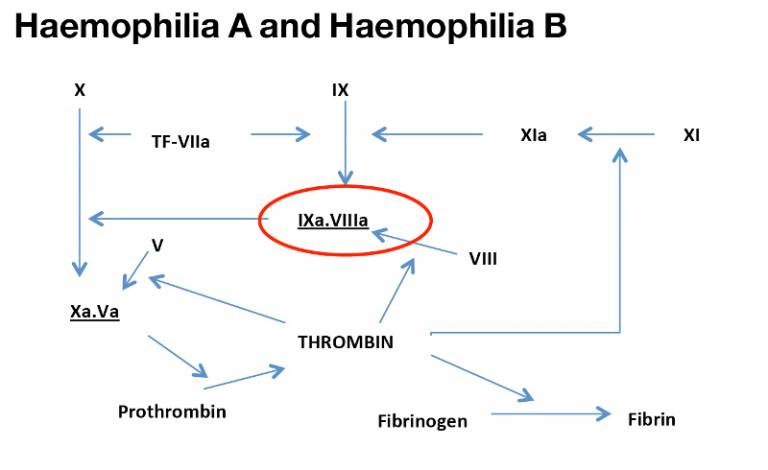
Current treatment is to use replacement therapy on an on-demand or prophylaxis basis to maintain levels above 1% of normal and protect joints from spontaneous bleeding. However, treatment is hampered by the frequency of administration up to three times per week intravenously and the high cost ~£100,000 per patient per year.
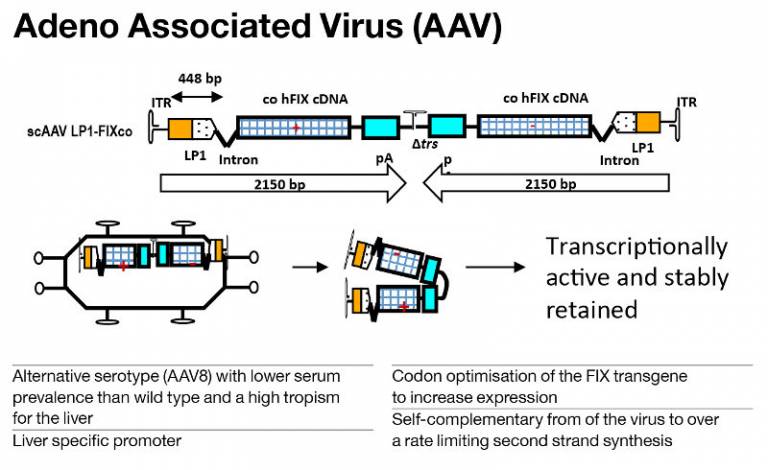
We use the adeno associated virus (AAV) to deliver the FIX transgene. AAV is a helper dependent virus and non-pathogenic and provides long term expression in post-mitotic tissues. We have changed some key aspects to improve expression.
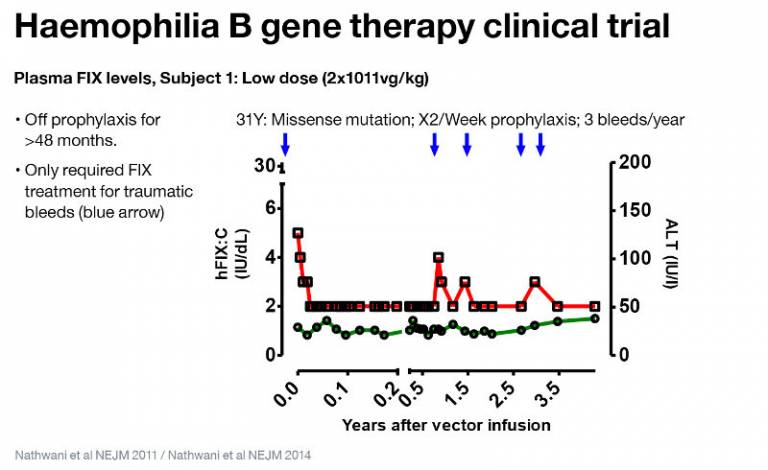
A total of ten patients have been treated in this phase I/II clinical trial. The first phase was a dose escalation of 2 x 1011, 6 x 1011, 2 x 1012 vg/kg, treating two patients before proceeding to the next dose level. The second phase was to increase the high dose cohort to a total of six treated patients.

This study has now been able to follow the first phase for more than three years, and has shown a long term expression of FIX levels of between 1–6%, with a mean level of 5.1% in the high dose cohort, resulting in a reduction of 90% in both bleeding episodes and FIX concentrate usage.
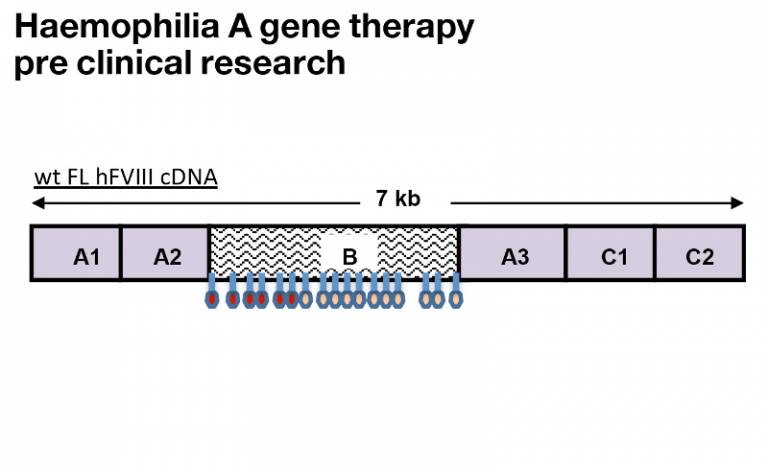
Our haemophilia A approach is based on our success with haemophilia B, using the same serotype and codon optimisation of the transgene. However, due to size constraints the vector is single stranded and a minimal liver specific promoter and a synthetic promoter have been used.
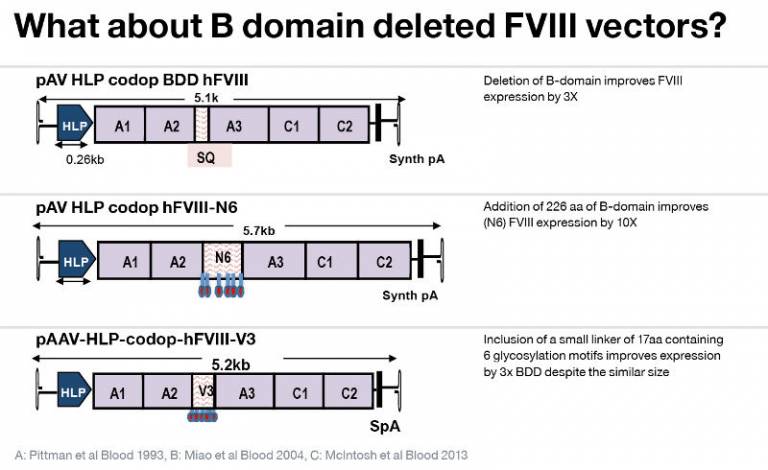
Major hurdles to safe and effective Haemophilia A gene therapy with AAV: FVIII gene specific: 4 fold lower expression than similar sized proteins. AAV specific: Limited packaging capacity
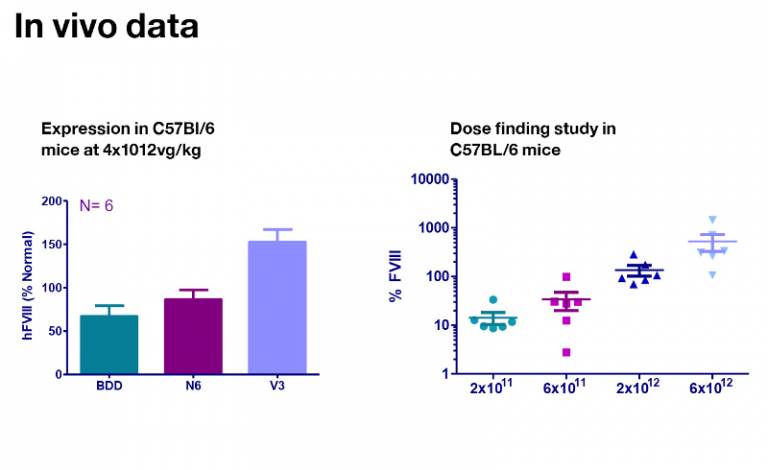
We will be using the pAAV HLP-codop-hFVIII-V3 construct in a forthcoming clinical trial using doses of 2x1011, 6x1011, 2x1012vg/kg.
 Close
Close

