Molecular mechanism of antigen retention in immune response unveiled
13 July 2023
Research led by UCL and the Francis Crick Institute has identified the biological mechanisms by which antigens are captured, stored and replaced in lymph nodes after vaccination and how they regulate B cell and antibody responses.
The findings, published today in Nature Immunology, provide valuable insight into human immune responses and point the way to understanding how the immune system could become better equipped to recognise and eliminate specific pathogens - leading to more efficient and targeted immune responses and vaccine design.
B cell bodyguards
B cells play a crucial role in the immune system. They are responsible for making special proteins called antibodies that specifically target and fight against harmful invaders, like germs and viruses.
When our immune system detects the presence of a pathogen (such as a virus or bacteria), or receives a vaccine, it activates B cells that are capable of recognising and binding to specific molecules on the surface of the pathogen, known as antigens. These activated B cells migrate to specific regions in the lymph nodes, called Germinal Centres (GCs), where they change and multiply to produce antibodies to attack the pathogen.
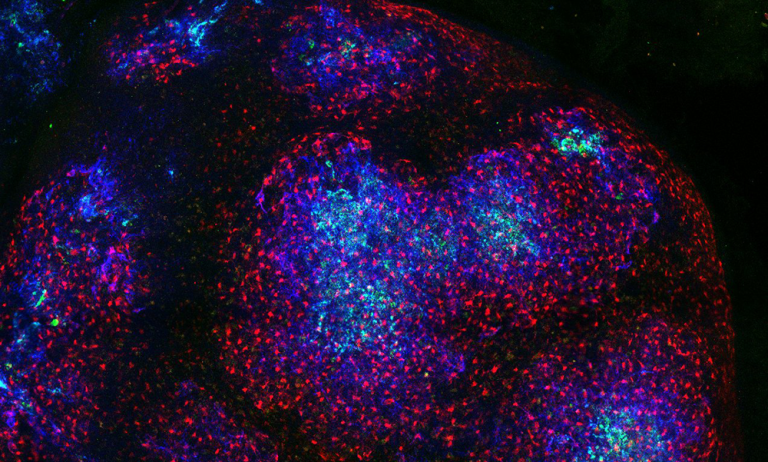
Lymph node GCs need a continuous supply of antigens to optimise the production of antibodies in response to germs and vaccines. To enable this, there is specialised network of cells - the Follicular Dendritic Cell network (FDC) - within the lymph node that captures antigens and acts as a reservoir, supplying the antigens to the GCs. The research team wanted to investigate the activity of this cellular network to understand more about how antigens were being retained and ‘presented’ to B cells to produce antibodies.
The vanguard of next generation vaccines
Their findings showed that the structure and arrangement of the FDC network made a difference in how antigens were captured and used. The team identified that only the cells in the centre of the network functioned as an antigen reservoir, whereas those on the periphery did not, and the molecular mechanisms underpinning this. This discovery has implications for the potential development of more efficient vaccines.
“The basic idea is that when you inject a vaccine, it needs to stay in the lymph nodes for some time for the B cell response to develop high quality antibodies. And so, if we understand how the antigen is retained actively in these immune sites, we might be able to manipulate that. Indeed, there's a great interest in designing vaccines in a way that will promote retention on these follicular dendritic cells that we are looking at,” explains Dr Pavel Tolar, lead author of the study and Professor of B Cell Immunology, UCL Institute of Immunity and Transplantation.
“There are several ways in which we might be able to manipulate antigen retention, for example by engineering nanoparticles with proteins that bind to FDCs, or an escalated dosing regimen that leads to long term deposition of antigens on these cells. Ultimately, what we are thinking about is how do we make a vaccine that works efficiently? If the vaccine material just passes through the lymph nodes, eventually it will be degraded and expelled from the body. The retention mechanism is an effective way of using the vaccine material (antigen) in the right place of the body for longer, you can inject less and still get a highly effective, and long-lasting, response,” says Dr Tolar.
Further information
- Research Paper: Long-term retention of antigens in germinal centers is controlled by the spatial organization of the follicular dendritic cell network. Nature Immunology
- Dr Pavel Tolar academic profile
- Tolar Lab - Division of Infection and Immunity
- Main Image: Image of a lymph node with the Follicular Dentrtic Cells in blue, all stroma in red and antigen in green. Credit UCL Pavel Tolar
 Close
Close

