Women And Newborn Trial of Antenatal Interventions and Management
Project Summary
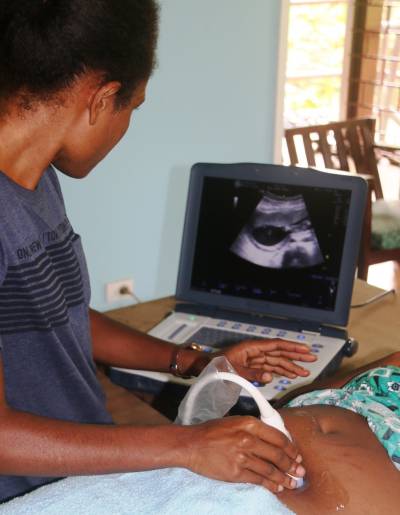
Women in many low-income countries worldwide face a high and unacceptable burden of adverse pregnancy outcomes.
Curable, sexually transmitted and genital infections (STIs), such as chlamydia, gonorrhoea, trichomonas and bacterial vaginosis, are major contributors to this disease burden but the majority of infections go untreated because most infections are asymptomatic, and affordable, easy to use and accurate diagnostic tests are unavailable in such settings. At the same time, there is conflicting evidence on the potential risks and benefits of STI screening and treatment in pregnancy, hindering policy and practice and leading to calls for definitive field trials.
The aim of the WANTAIM Trial is to measure the effectiveness, health system requirements, cost-effectiveness and acceptability of antenatal point-of-care testing and treatment of sexually transmitted and genital infections to improve birth outcomes in high-burden, low-income settings.
 Close
Close

