MCL studies fundamentals of bond breaking and formation process for emission control and energy storage. Two fully funded PhD position available for 2022/23, please contact ryan.wang@ucl.ac.uk

About
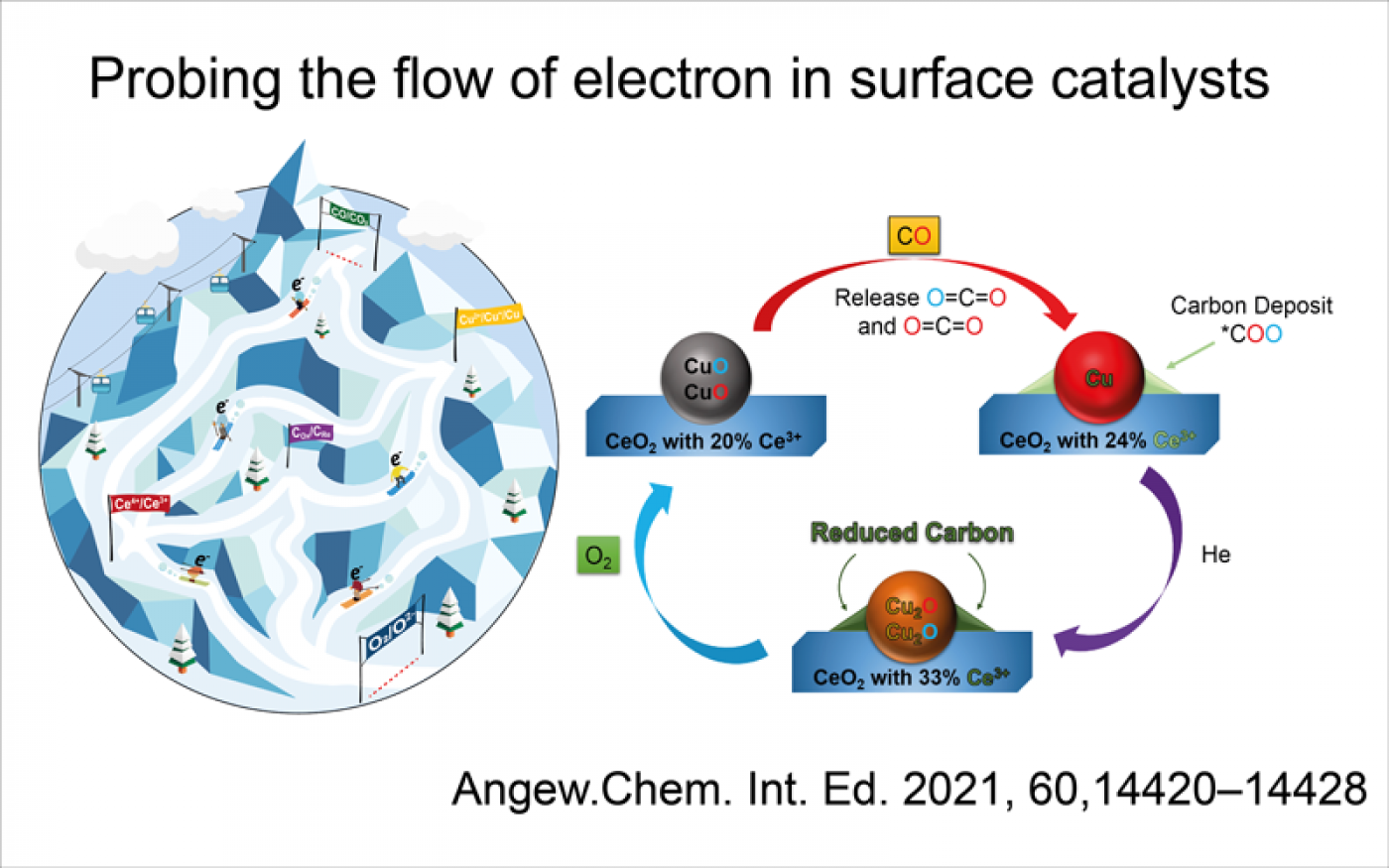
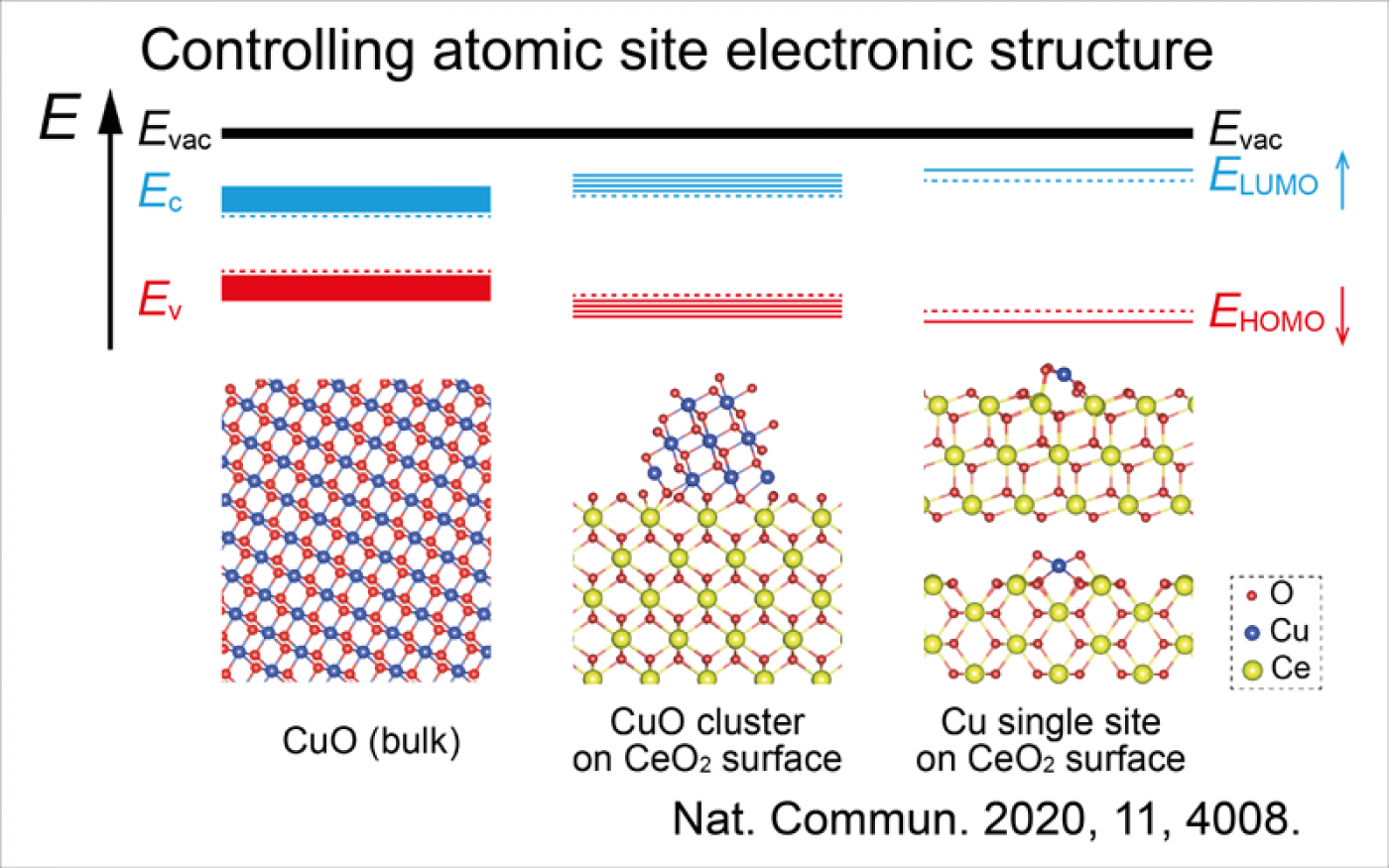
We specialize in operando spectroscopy, studying the catalyst properties under the realistic reaction condition. This enables the direct observation of bond breaking and formation dynamics, in particular in oxidation reaction with molecular oxygen. We have published a few papers at top tier journals (Nat. Comm., Angew. Chem., J. Am. Chem. Soc.) to show our leading position in this field. Our uniqueness is pushing the envelop of the operando techniques in order to unveil the hiding dynamics and mechanism of oxidation reaction, namely “Faster, Higher, Strong”.
Faster: With faster X-ray pulses, we can detect bond breaking and formation dynamics on surface and in solution, without measuring the diffusion and adsorption of reaction molecules. This is only possible with free electronlasers (FEL). We are the first UK user to perform such X-ray FEL (XFEL) experiment in catalysis.
Higher: With the higher beam energy and intensity, it is then possible to record spectra with low emission probability. Those emission lines are highly related to the valence states, and are therefor more sensitive to the bondbreaking and formation.
Stronger: With a stronger and more focused beam, it is then possible to achieve high space resolution toward nm region, providing spatial resolved XAFS/XRF imaging on target locations. This is then correlated with electron imaging (EM) to provide statistical understanding of the catalyst performance. We have used this to image the materials degradation in catalysis.
Most of those studies are carried out at synchrotron and XFEL facilities around world, including the Diamond Light Source, BESSY II, Spring-8, PetraIII, TPS and PAL-XFEL. The improvement of X-ray techniques enables better understanding of bonding chemistry.
 Close
Close

