Scientists develop new catalyst material for longer-lasting fuel cells
27 July 2020
In the study, published last week in the journal Nanoscale, researchers produced graphene via a scalable technique and used it to develop a hydrogen fuel cell catalyst.
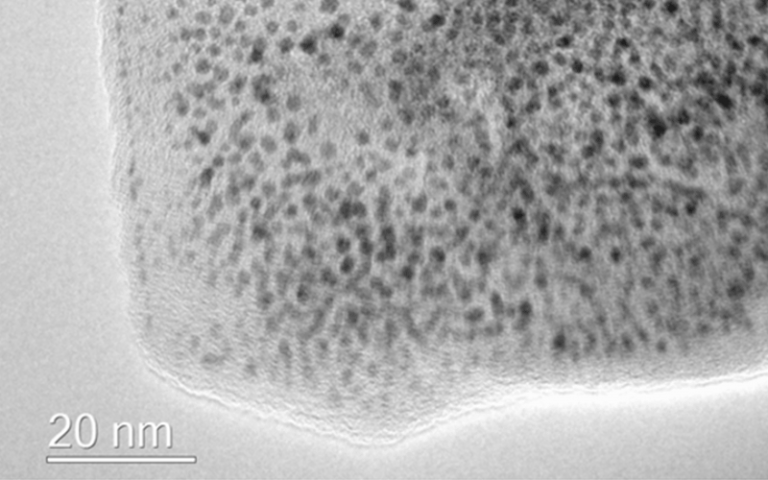
They showed that this new type of graphene-based catalyst was more durable than commercially available catalysts and matched their performance. Hydrogen fuel cells convert chemical energy into electrical power by combining hydrogen and oxygen with the aid of catalysts. As the only by-product of the reaction is water, they provide an efficient and environmentally friendly power source. Platinum is the most widely used catalyst for these fuel cells, but its high cost is a big problem for the commercialisation of hydrogen fuel cells.
To address this issue, commercial catalysts are typically made by decorating tiny nanoparticles of platinum onto a cheaper carbon support, however the poor durability of the material greatly reduces the lifetime of current fuel cells. Previous research has suggested graphene could be an ideal support material for fuel cells due to its corrosion resistance, high surface area and high conductivity. However, the graphene used in the majority of experiments to-date contains many defects, meaning that the predicted improved resistance has not yet been achieved. The technique described in the study produces high-quality graphene decorated with platinum nanoparticles in a one-pot synthesis. This process could be scaled up for mass production, opening up the use of graphene-based catalysts for widespread energy applications.
“Professor Dan Brett, Professor of Electrochemical Engineering at UCL, said: "Satisfying global energy demands without damaging the environment is one of the great modern challenges. Hydrogen fuel cells can provide cleaner energy and are already used in some cars as an alternative to petrol or diesel. However, a big barrier to their widespread commercialisation is the ability for catalysts to withstand extensive cycling required for their use in energy applications. We’ve shown that by using graphene instead of the typical amorphous carbon as a support material we can create ultra-durable catalysts."
Further Information
- Research publication: ‘Realising the electrochemical stability of graphene: scalable synthesis of ultra-durable platinum catalyst for the oxygen reduction reaction’ Gyen Ming A. Angel, Noramalina Mansor, Rhodri Jervis, Zahra Rana, Chris Gibbs, Andrew Seel, Alexander F.R. Kilpatrick, Paul R. Shearing, Christopher A. Howard, Dan J. L. Brett and Patrick L. Cullen, Nanoscale.
Image Credit:
- Patrick Cullen / Gyen Ming Angel
 Close
Close


