New study from CNIE published in the Journal of the American Chemical Society
8 October 2018
Small-angle neutron scattering (SANS) reveals stabilization of fluid-like protein arrangements in mesoporous silica, facilitated by geometry-dependent crowding effects
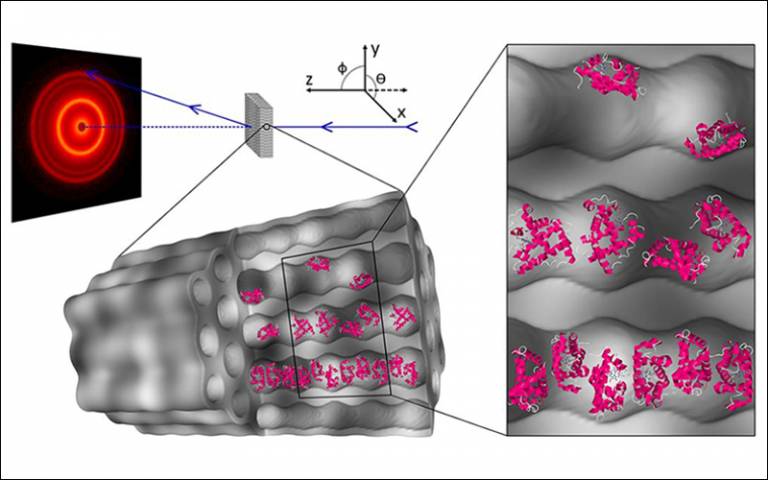
A new paper titled, 'Confinement Facilitated Protein Stabilization As Investigated by Small-Angle Neutron Scattering', was published in the Journal of the American Chemical Society.
CNIE's Justin Siefker and Prof Marc-Olivier Coppens co-authored the paper in collaboration with researchers from Forschungszentrum Jülich.
Authors: Justin Siefker, Ralf Biehl, Margarita Kruteva, Artem Feoktystov and Marc-Olivier Coppens.
About the paper
While mesoporous silicas have been shown to be a compelling candidate for drug delivery and the implementation of biotechnological applications requiring protein confinement and immobilization, the understanding of protein behavior upon physical adsorption into silica pores is limited. Many indirect methods are available to assess general adsorbed protein stability, such as Fourier-transform infrared spectroscopy and activity assays. However, the limitation of these methods is that spatial protein arrangement within the pores cannot be assessed. Mesoporous silicas pose a distinct challenge to direct methods, such as transmission electron microscopy, which lacks the contrast and resolution required to adequately observe immobilized protein structure, and nuclear magnetic resonance, which is computationally intensive and requires knowledge of the primary structure a priori.
Small-angle neutron scattering can surmount these limitations and observe spatial protein arrangement within pores. Hereby, we observe the stabilization of fluid-like protein arrangement, facilitated by geometry-dependent crowding effects in cylindrical pores of ordered mesoporous silica, SBA-15. Stabilization is induced from a fluid-like structure factor, which is observed for samples at maximum protein loading in SBA-15 with pore diameters of 6.4 and 8.1 nm. Application of this effect for prevention of irreversible aggregation in high concentration environments is proposed.
 Close
Close

