Group Leader: Dr Surinder Sharma
Research
The focus of our research is to develop and optimise the antibody directed enzyme prodrug therapy (ADEPT) for cancer as a single entity and in combination with other agents which may give synergy. The main goal is successful development and translation of rationally designed therapy strategies from in-vitro to in-vivo models and clinical application for the benefit of people with cancer. To support the clinical studies, the group designs bioanalytical assays, develops these into validated methods and implements them in compliance with good clinical laboratory practice (GCLP).
ADEPT
The objective of antibody directed enzyme prodrug therapy (ADEPT) is to selectively deliver chemotherapy to cancer sites. The basic principle of ADEPT (Fig 1) is to target an enzyme to tumours by attaching it to an antibody directed to a tumour associated antigen. After clearance of enzyme from blood, a non-toxic prodrug is given. The targeted enzyme converts a non-toxic prodrug into a potent cell killing agent within tumours to achieve effective therapy without normal tissue toxicity.
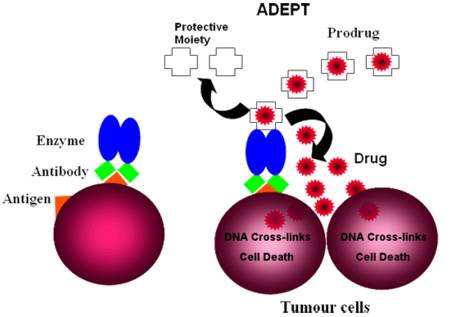
Figure 1. The basic principle of ADEPT.
Pre-Clinical studies
I have been involved with ADEPT since its inception in the 1980’s and since then have been responsible for the design and execution of all the pre-clinical studies using in-vivo models. These studies have confirmed the theoretical considerations that underpin the ADEPT concept. In particular, the necessity for removing enzyme activity in blood by inactivation of the enzyme or by its rapid clearance before the prodrug is given. These studies have been the basis for 4 ADEPT clinical trials. The selective delivery of enzyme to tumours with rapid clearance from blood has been achieved both in in-vivo models (Fig 2A and 2B) and in patients (Fig 2C). A single ADEPT cycle produces growth delay in in-vivo models, while multiple ADEPT cycles produce tumour regressions in both pre-clinical (Fig 3A) and clinical trials (Fig 3B).
We are currently developing enhanced ADEPT systems incorporating the original three phase concept. This involves inactivation and removal of enzyme activity from blood and other non-tumour sites prior to prodrug administration so that toxicity to normal tissue is avoided. To achieve this, a library of novel clearing agents in the form of glycosylated peptidomimetic molecules has been created. These molecules can bind to the active site of the enzyme resulting in specific inhibition of the enzyme activity and subsequent clearance of the complex via the asialoglycoprotein receptor.
In vitro and in vivo studies are in progress to select suitable candidates for enhanced ADEPT.
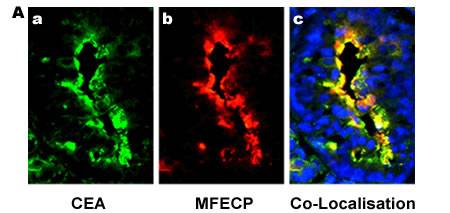
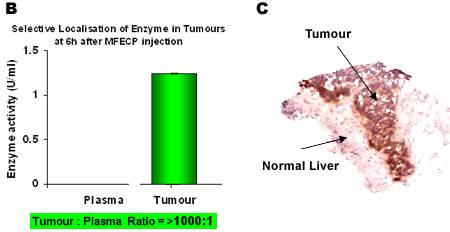
Figure 2. Fluorescence images of human colon carcinoma cells showing selective localisation of antibody-enzyme fusion protein (MFECP) in-vivo.
Panel A: a) Expression of CEA, b) Localisation of i.v. given MFECP fusion protein, c) Co-localisation of CEA and MFECP (yellow) and DAPI (blue) showing tumour cell nuclei (x 400 magnification). [Sharma et al, Clin Cancer Res., 2005]
Panel B: Selective retention of enzyme activity in tumour and rapid clearance of enzyme activity from blood with MFECP in-vivo.
Panel C. A liver biopsy specimen from an ADEPT patient showing localisation of antibody-enzyme (MFECP) in metastatic tumour cells. [Mayer et al, Clin Cancer Res., 2006]
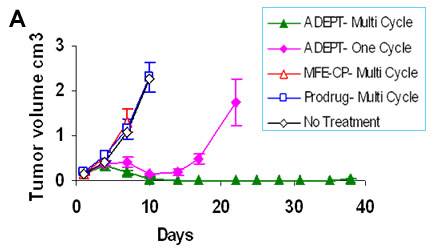
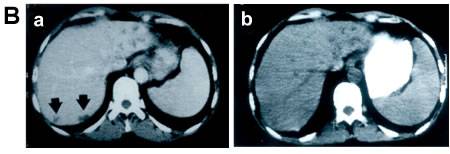
Figure 3.
Panel A: Multiple cycles of ADEPT result in tumour regression in in-vivo.
Panel B: a) Metastatic tumour deposits of colorectal cancer in Liver (arrows) before treatment and b) tumour regression after ADEPT treatment [Napier et al, Clin Cancer Res.,2000]
Clinical Trials - ADEPT
The group has critical responsibilities in relation to clinical trials. We develop improved techniques for laboratory measurement of parameters that are important to the care, safety and management of patients. One essential feature of the ADEPT clinical trials has been the need to monitor patient’s blood enzyme levels to ensure that prodrug is given only when it is safe to do so. These measurements are performed by the group in real time.
Other clinical trials
We also perform pharmacokinetic and pharmacodynamic measurements that are an essential component of all Phase I/II clinical trials that are undertaken by the department. These clinical studies include a diverse range of molecules including radiolabelled monoclonal, single chain Fv and chimeric antibodies, as well as combination therapies with anti-vascular agents. In addition, laboratory support is provided for any external Phase II/III studies.
Immunogenicity of Biologics
During the course of therapy with biologics, patients may develop an unwanted immune response to the therapeutic which may affect efficacy and safety. Therefore, the detection of immune response is an essential component of the pre-clinical and clinical studies with antibody-based molecules. In repeated cycles of treatment with radioimmunotherapy or ADEPT, we monitor patients for immune response to the antibody or antibody-enzyme constructs, in real time as the appearance of antibodies to the therapeutic determines the end point of treatment.
We have applied a number of approaches to reduce the immunogenicity of our molecules e.g. identification the immunodominat B cell epitopes on the enzyme and use of immunosuppressive agents. The immunogenicity of engineered new molecules is also studied in-vivo (Fig 4). New immunosuppressive agents as well as human enzymes are also been investigated. We apply a risk-based approach to the assessment and management of immunogenicity in our clinical trials. The group develops an appropriate assay strategy for measurement and confirmation of antibodies directed to the therapeutic. Neutralisation assays are also being developed.
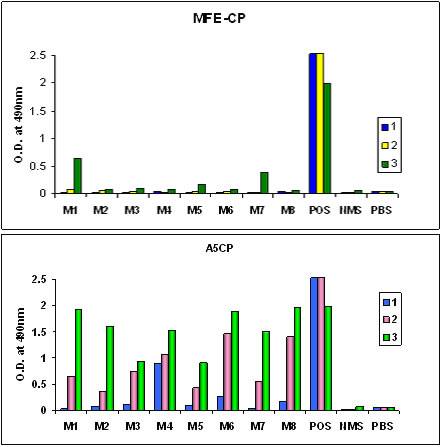
Figure 4. Immunogenicity in-vivo.
Second generation recombinant antibody-enzyme fusion protein (MFECP) showing reduced immunogenicity of enzyme [2/8 (M1 and M7) serum samples positive for anti-enzyme antibody] compared to the first generation chemically conjugated antibody-enzyme molecule [8/8 serum samples positive for anti-enzyme antibody].
DNA Damage and Repair
The prodrugs utilised in our ADEPT studies are converted to bi-functional alkylating agents by the action of the targeted enzyme. The cytotoxicity of these drugs results from drug-induced cross-links in the tumour cell DNA (Fig 5). As these cross-links may be removed by various repair mechanisms, the rate of repair is of importance for therapeutic efficacy. We are investigating the DNA damage and repair effects related to ADEPT and other antibody based therapies, in vitro and in-vivo. A range of agents that inhibit tumour cell DNA repair are being explored. Tumour parameters related to therapeutic response, e.g. gamma H2AX formation, cell proliferation and apoptosis, are being investigated using quantitative, high resolution microscopy.
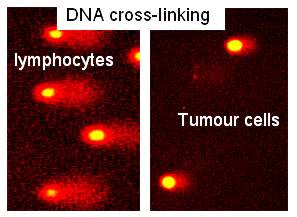
Figure 5. Effect of ADEPT.
A biopsy showing DNA damage in Tumour cells (short Comet tail). No DNA damage within normal blood lymphocytes (long Comet tail).
Combination Therapies
We are currently exploring new combination therapies to enhance tumour cell death without affecting the normal tissues. These include studies of new prodrugs, inhibitors of DNA repair, anti-vascular, anti-angiogenic agents as well as and complimentary antibodies, which may synergise with ADEPT for the treatment of a variety of different tumour types.
Rationally designed successful combinations will then be studied in novel clinical trials.
GCLP Compliance
We lead the CRUK T & I group’s quality assurance programme for Good Clinical Laboratory Practice (GCLP), and closely liaise with Cancer Research-UK Drug development Office and the Quality Assurance management. The conditions for collecting, handling, processing and storing biological samples are standardized along with the appropriate training of clinical trial personnel to ensure the stability and integrity of analyte. We develop bioanalytical assays using different technologies to support pharmacokinetic and pharmacodynamic studies of therapeutic agents as well as biomarkers related to clinical trials. The appropriate assay is developed into a validated method which is then eligible for clinical trial application.
Selected publications
Sharma, S. K. and Bagshawe, K. D. (2017) Translating antibody directed enzyme prodrug therapy (ADEPT) and prospects for combination. Expert Opin Biol Ther. 2017 Jan;17(1):1-13. doi: 10.1080/14712598.2017.1247802
Sharma, S. K., Chester, K. A. and Bagshawe, K. D. (2014) Antibody-Directed Enzyme Prodrug Therapy (ADEPT), in Handbook of Therapeutic Antibodies (eds S. Dübel and J. M. Reichert), Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, Germany. doi: 10.1002/9783527682423.ch18
Sharma, S. K. (2013) Antibody-Directed Enzyme Prodrug Therapy (ADEPT), in Fusion Protein Technologies for Biopharmaceuticals: Applications and Challenges (ed S. R. Schmidt), John Wiley & Sons, Inc., Hoboken, NJ, USA. doi: 10.1002/9781118354599.ch23
Andrady C, Sharma S.K., Chester KA. Antibody-enzyme fusion proteins for cancer therapy. Immunotherapy. 2011 Feb;3(2):193-211. Review. Pubmed PMID: 21322759
Sharma S.K., Pedley RB. In Antibody Engineering; Xenograft Mouse Models for Tumour Targeting. Springer Protocols. 2010, 477-490.
Begent R, Sharma S.K., Chester K. In Antibody Engineering; Antibody-Dependent Enzyme Prodrug Therapy (ADEPT). Springer Protocols. 2010, 431-451. DOI: 10.1007/978-3-642-01144-3_28
Dancey G, Violet J, Malaroda A, Green AJ, Sharma SK, Francis R, Othman S, Parker S, Buscombe J, Griffin N, Chan PS, Malhotra A, Woodward N, Ramsay A, Ross P, Lister TA, Amlot P, Begent R, McNamara C. A Phase I Clinical Trial of CHT-25 a 131I-Labeled Chimeric Anti-CD25 Antibody Showing Efficacy in Patients with Refractory Lymphoma. Clin Cancer Res. 2009 Dec 15;15(24):7701-7710. PubMed PMID: 20008855.
Meyer T, Gaya AM, Dancey G, Stratford MR, Othman S, Sharma SK, Wellsted D, Taylor NJ, Stirling JJ, Poupard L, Folkes LK, Chan PS, Pedley RB, Chester KA, Owen K, Violet JA, Malaroda A, Green AJ, Buscombe J, Padhani AR, Rustin GJ, Begent RH. A phase I trial of radioimmunotherapy with 131I-A5B7 anti-CEA antibody in combination with combretastatin-A4-phosphate in advanced gastrointestinal carcinomas. Clin Cancer Res. 2009 Jul 1;15(13):4484-92. Epub 2009 Jun 23. PubMed PMID: 19549771.
Yong M, Tolner B, Nagl S, Pedley RB, Chester K, Green AJ, Mayer A, Sharma S, Begent R. Data standards for minimum information collection for antibody therapy experiments. Protein Eng Des Sel. 2009 Mar;22(3):221-4. PubMed PMID: 19224941.
Tolner B, Smith L, Hillyer T, Bhatia J, Beckett P, Robson L, Sharma S K, Griffin N, Vervecken W, Contreras R, Pedley RB, Begent RH, Chester KA. From laboratory to Phase I/II cancer trials with recombinant biotherapeutics. Eur J Cancer 2007 Nov;43(17):2515-22.
Kogelberg H, Tolner B, Sharma SK, Lowdell MW, Qureshi U, Robson M, Hillyer T, Pedley RB, Vervecken W, Contreras R, Begent RH, Chester KA (2007) Clearance mechanism of a mannosylated antibody-enzyme fusion protein used in experimental cancer therapy. Glycobiology 17: 36-45
Mayer A, Francis RJ, Sharma SK, Tolner B, Springer CJ, Martin J, Boxer GM, Bell J, Green AJ, Hartley JA, Cruickshank C, Wren J, Chester KA, Begent RH (2006) A phase I study of single administration of antibody-directed enzyme prodrug therapy with the recombinant anti-carcinoembryonic antigen antibody-enzyme fusion protein MFECP1 and a bis-iodo phenol mustard prodrug. Clin Cancer Res 12: 6509-6516
Sharma SK, Pedley RB, Bhatia J, Boxer GM, El-Emir E, Qureshi U, Tolner B, Lowe H, Michael NP, Minton N, Begent RH, Chester KA (2005) Sustained tumor regression of human colorectal cancer xenografts using a multifunctional mannosylated fusion protein in antibody-directed enzyme prodrug therapy. Clin Cancer Res 11: 814-825
 Close
Close

