David Attwell
Brain Energy Use
We pioneered analyses of the subcellular tasks on which the brain uses energy, establishing the first energy budgets for the grey and white matter of the brain (Attwell & Laughlin, 2001; Harris & Attwell, 2012), which were calculated from the bottom up, from the measured properties of ion channels, synapses, cell anatomy and action potential rates. We showed that brain energy use imposes profound constraints on the speed with which the brain can process information, dictating that energy-saving coding and information transmission strategies must be used. We also demonstrated that this implies that the energy supply to the brain is unexpectedly linked to parameters determining the speed with which it operates, including the affinity of glutamate receptors, the diameter of synaptic boutons and the rate of binding of glutamate to its transporters (Attwell & Gibb, 2005).
The energy budget analysis showed that most brain energy is used postsynaptically at synapses. This requires that ATP be made postsynaptically at active synapses. In collaboration with Josef Kittler, we have studied how the mitochondria which make this ATP are located at active synapses. This occurs by virtue of calcium entry through postsynaptic NMDA receptors uncoupling the mitochondrial adaptor protein Miro from the motor protein which moves mitochondria along microtubules.
Analysis of how information transfer through synapses depends on the number of postsynaptic receptors (performed experimentally using dynamic clamp: Harris et al., 2015) and on presynaptic release probability (Harris et al., 2012) revealed that both of these parameters are optimised to maximise the rate at which information is transmitted per energy used (rather than optimised purely to maximise information transmission).
The remarkably low release probability of central synapses was thus predicted to result from the observed degree of convergence of synapses from a single presynaptic axon onto a postsynaptic cell.
Astrocytes, neurotransmitter transporters and stroke
Astrocytes have been suggested to release gliotransmitters onto neurons, thus regulating their excitability and synaptic strength (Bazargani & Attwell, 2016). We are currently investigating how a previously unstudied G protein coupled receptor on astrocytes mediates this effect (Jolly, Bazargani et al., 2017), and how amine transmitters regulate astrocyte function (Bazargani & Attwell, 2017).
Astrocytes also play a key role in regulating the excitability of neurons, and preventing excitotoxic damage, by using transporters to control the extracellular glutamate level. We pioneered the use of patch-clamp techniques to study transporters for the main excitatory neurotransmitter glutamate (Brew & Attwell, 1987), investigating in particular the ion movements which drive glutamate transport (Barbour, Brew & Attwell, 1988, Bouvier et al., 1992; Levy et al., 1998).
We have demonstrated that glutamate transporters can run backwards when ion gradients run down in conditions like stroke (Szatkowski, Barbour & Attwell, 1990; Attwell et al., 1993), releasing enough glutamate to activate receptors in nearby neurons (Billups & Attwell, 1996).
Indeed, in the first 10 minutes of a stroke, reversed operation of glutamate transporters is the main mechanism by which the extracellular glutamate concentration of glutamate is raised to levels which trigger the death of neurons, leading to mental and physical impairment (Rossi, Oshima & Attwell, 2000; Szatkowski & Attwell, 1994).
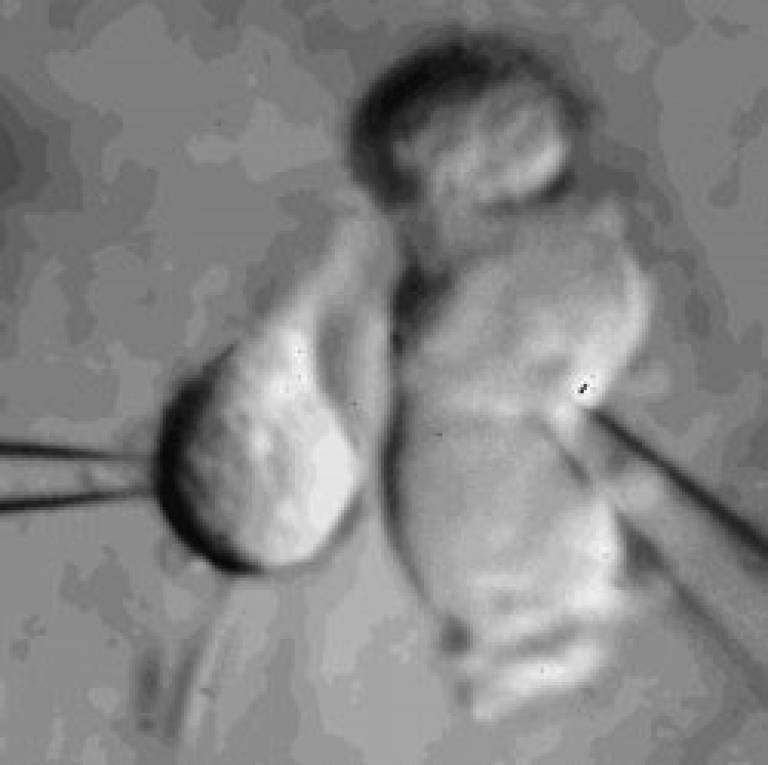

Microglia
Microglial cell in a brain slice constantly extending and retracting its processes to survey the environment. This surveillance is thought to be important for detecting invading organisms, assessing the function of and pruning synapses, and regulating the activity of neurons. Scale bar 20 microns; movie duration 30 minutes.
Our recent work on microglia has identified a potassium channel that regulates cell ramification and surveillance of the brain (Madry et al., 2017).
Future work will investigate how modulation of this channel affects synapse pruning (Izquierdo et al., 2021), animal behaviour and the response to pathology.
Oligodendrocytes and the node of Ranvier
Our work on oligodendrocytes covers the development, plasticity and pathology of these cells. Oligodendrocytes myelinate axons, and thus speed action potentials, but in pathological conditions like cerebral palsy, stroke, spinal cord injury and multiple sclerosis oligodendrocytes are killed, leading to mental and physical impairment.
We have shown that neurotransmitter receptors for glutamate and GABA regulate the development of oligodendrocyte precursor cells into myelinating oligodendrocytes (Káradóttir et al., 2005; Lundgaard et al., 2013; Hamilton et al., 2016; Kougioumtzidou et al., 2017).
Current work is focused on how the length of the node of Ranvier is set, how it affects conduction speed, and whether it can be changed in situations where neuronal circuitry needs to be plastic to alter behaviour (Arancibia-Carcamo et al., 2017).
For pathology, we recently discovered that a damaging rise of calcium concentration in the myelin sheath that occurs in ischaemia is produced by intracellular protons activating TRP channels (Hamilton et al., 2016).
Ischaemic pathology also damages the node of Ranvier, and we are characterising the mechanisms by which this occurs.
Pericytes, and control of brain energy supply
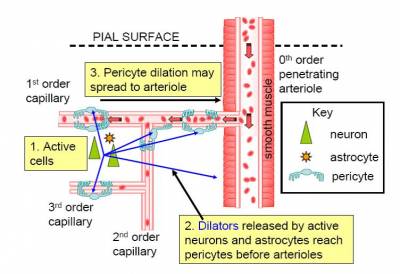
Although brain blood flow is often thought to be controlled by the smooth muscle around arterioles (Attwell et al., 2010), there are contractile cells (pericytes) at roughly 30 micron intervals along capillaries.
In the brain, unlike most other tissues, it turns out that most of the vascular resistance is located in capillaries rather than in arterioles, so the adjustment of capillary diameter by these cells can strongly affect blood flow. We have shown that capillary pericytes respond to messengers generated by neurotransmitter glutamate (such as prostaglandin E2) and that they respond more rapidly than arterioles to increases of neuronal activity (Peppiatt et al., 2006; Hall et al., 2014).
They also generate the majority of the blood flow rise evoked by neuronal activity, and thus may be the main driver of the BOLD fMRI signals that are used to non-invasively probe brain function by psychologists. Interestingly, we have recently found that dilation of capillaries is evoked by a different signalling pathway from dilation of arterioles: capillary dilation reflects neuronal ATP release evoking a rise of calcium concentration in astrocytes which then triggers the release of prostaglandin E2, while arteriole dilation does not seem to involve ATP release or astrocyte calcium, and may be driven mainly by NO generated in interneurons (Mishra et al., 2016).
Pericytes play a key role in brain ischaemia. Work from the 1960s showed that after brain ischaemia the microvasculature is not properly reperfused even if the causative clot is removed from an artery to the brain. This restriction of energy supply will lead to ongoing damage to neurons. Imaging pericytes during ischaemia showed that they constrict capillaries (Peppiatt et al., 2006), presumably because the loss of ATP supply inhibits their ion pumping, leading to a rise of intracellular calcium concentration. Further work demonstrated that the reason that the decrease of blood flow is so long lasting is that pericytes die readily, in rigor, leaving the capillaries constricted even if the energy supply is restored (Hall et al., 2014). Similar events occur in the heart after cardiac ischaemia (O'Farrell et al., 2017).
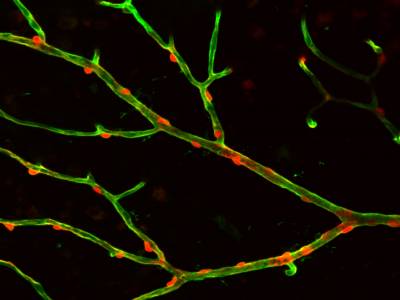
Movie showing that depolarisation of a pericyte constricts the retinal capillary on which it is located, and this is followed by constriction of the pericytes on each side of the stimulated cell.
Translating our work into human therapy
We are keen to translate our work on rodents to generate treatments that benefit humans. To this end, we are further investigating how our discovery that TRP channels generate a deleterious calcium influx into oligodendrocytes (Hamilton et al., 2016) can be employed to reduce white matter damage in multiple sclerosis, stroke, heart attack, Alzheimer's disease and spinal cord injury. In addition, as outlined above, pericytes are a therapeutic target in stroke, and we have already discovered a drug which prevents them constricting capillaries and dying in ischaemia.
As an important step towards ensuring that discoveries made in rodent tissue are relevant to human therapy, we have started to study pericytes in living slices of human brain. The tissue is obtained when it is necessary clinically to remove a small piece of normal brain tissue in order to access an underlying brain cancer. Normally this removed tissue would be discarded but, with informed consent from the patients who generously donate the tissue, ethical approval, and the generous help of the neurosurgeon Huma Sethi who carries out the operations, we are able to take the tissue and study it in the lab.
The results obtained to date show that, like rodent pericytes, human capillary pericytes constrict in response to noradrenaline and dilate in response to glutamate (Nortley et al., 2019). Currently we are investigating the response of the human pericytes to ischaemia.
Lab Members
Selected Publications
Lezmy J, Arancibia-Cárcamo IL, Quintela-López T, Sherman DL, Brophy PJ, Attwell D. (2021) Astrocyte Ca2+ -evoked ATP release regulates myelinated axon excitability and conduction speed. Science 374, eabh2858. DOI: 10.1126/science.abh2858.
Nortley R, Korte N, Izquierdo P, Hirunpattarasilp C, Mishra A, Jaunmuktane Z, Kyrargyri V, Pfeiffer T, Khennouf L, Madry C, Gong H, Richard-Loendt A, Huang W, Saito T, Saido TC, Brandner S, Sethi H, Attwell D. (2019) Amyloid oligomers constrict human capillaries in Alzheimer's disease via signaling to pericytes. Science 365, 250 DOI: 10.1126/science.aav9518.
Krasnow, A.M., Ford, M.C., Valdivia, L.E., Wilson, S.W. & Attwell, D. (2018) Regulation of developing myelin sheath elongation by oligodendrocyte calcium transients in vivo. Nature Neurosci. 21, 24-28.
Jolly, S., Bazargani, N., Quiroga, A.C., Pringle, N.P., Attwell, D., Richardson, W.D. & Li, H. (2018) G protein-coupled receptor 37-like 1 modulates astrocyte glutamate transporters and neuronal NMDA receptors and is neuroprotective in ischemia. Glia 66, 47-61.
Madry, C., Kyrargyri, V., Arancibia-Cárcamo, I.L., Jolivet, R., Kohsaka, S., Bryan, R.M. & Attwell, D. Microglial Ramification, Surveillance, and Interleukin-1 Release Are Regulated by the Two-Pore Domain K+ channel THIK-1. Neuron. 2018 Jan 17;97, 299-312.
O'Farrell, F.M., Mastitskaya, S., Hammond-Haley, M., Freitas, F., Wah, W.R. & Attwell, D. (2017) Capillary pericytes mediate coronary no-reflow after myocardial ischaemia. Elife. 2017 Nov 9;6. pii: e29280. doi: 10.7554/eLife.29280.
Kougioumtzidou, E., Shimizu, T., Hamilton, N.B., Tohyama, K., Sprengel, R., Monyer, H., Attwell, D. & Richardson, W.D. (2017) Signalling through AMPA receptors on oligodendrocyte precursors promotes myelination by enhancing oligodendrocyte survival. Elife. 6, pii: e28080. Bazargani, N. & Attwell, D. (2017) Amines, Astrocytes, and Arousal. Neuron 94, 228-231.
Arancibia-Carcamo, I.L., Ford, M.C., Cossell, L., Ishida, K., Tohyama, K. & Attwell, D. (2017) Node of Ranvier length as a potential regulator of myelinated axon conduction speed. eLife e23329.
Mishra, A., Reynolds, J., Chen, Y., Gourine, A., Rusakov, D. & Attwell, D. (2016) Astrocytes mediate neurovascular signalling to capillary pericytes but not to arterioles. Nature Neuroscience 19, 1619-1627.
Hamilton, N.B., Kolodziejczyk, K., Kougioumtzidou, E. & Attwell, D. 2016) Proton-gated Ca2+-permeable TRP channels damage myelin in conditions mimicking ischaemia. Nature 529, 523-527.
Bazargani, N. & Attwell, D. (2016) Astrocyte calcium signalling: the third wave. Nature Neuroscience 19, 182-189.
Attwell, D., Mishra, A., Hall, C., O'Farrell, F. & Dalkara, T. (2016) What is a pericyte? J. Cereb. Blood Flow Metab. 36, 451-455.
Hamilton, N.B., Clarke, L., Arancibia-Carcamo, I.L., & Attwell, D. (2016) Endogenous GABA release regulates oligodendrocyte precursor cell proliferation, myelination, and internode length, Glia 65, 309-321.
Harris, J.J., Jolivet, R., Engl, E. & Attwell, D. (2015) Energy-efficient information transfer by visual pathway synapses. Current Biology 25, 3151-3160.
Ford, M.C., Alexandrova, O., Cossell, L., Stange-Marten,, A., Sinclair, J., Kopp-Scheinpflug, C., Pecka, M., Attwell, D. & Grothe, B. (2015)Tuning of Ranvier node and internode properties in myelinated axons to adjust action potential timing. Nature Commun. 6: 8073.
Hall, C.N., Reynell, C., Gesslein, B., Hamilton, N.B., Mishra, A., Sutherland, B., O'Farrell, F.M., Buchan, A.M., Lauritzen, M. & Attwell, D. (2014) Capillary pericytes regulate cerebral blood flow in health and disease. Nature 508, 55-60.
Arancibia-Carcamo, I.L. & Attwell, D. (2014) The node of Ranvier in CNS pathology. Acta Neuropathologica 128, 161-175.
O'Farrell, F.M. & Attwell, D. (2014) A role for pericytes in coronary no-reflow. Nat. Rev. Cardiol. 11, 427-432.
Lundgaard, I., Luzhynskaya, A., Stockley, J.H., Wang, Z., Evans, K.A., Swire, M., Volbracht, K., Gautier, H.O., Franklin, R.J., ffrench-Constant, C., Attwell D, Káradóttir RT. (2013) Neuregulin and BDNF induce a switch to NMDA receptor-dependent myelination by oligodendrocytes. PLoS Biol. 11, e1001743.
Young, K.M., Psachoulia, K., Tripathi, R.B., Dunn, S.J., Cossell, L., Attwell, D., Tohyama, K. & Richardson, W.D. (2013) Oligodendrocyte dynamics in the healthy adult CNS: evidence for myelin remodeling. Neuron 77, 873-885.
Harris, J.J., Jolivet, R. & Attwell, D. (2012) Synaptic energy use and supply. Neuron 75, 762-777.
Harris, J.J. & Attwell, D. (2012) The energetics of central nervous system white matter. J. Neurosci. 2, 356-371.
Attwell, D., Buchan, A.M., Charpak, C., Lauritzen, M., MacVicar, B.A. & Newman, E.A. (2010) Glial and neuronal control of brain blood flow. Nature 468, 232-243.
Hamilton, N.B. & Attwell, D. (2010) Do astrocytes really exocytose neurotransmitters? Nature Reviews Neuroscience 11, 227-238
MacAskill, A.F., Rinholm, J.E., Twelvetrees, A.E., Arancibia-Carcamo, I.L., Muir, J., Fransson, A., Aspenstrom, P., Attwell, D. & Kittler, J. (2009) Miro1 is a calcium sensor for glutamate receptor dependent localization of mitochondria at synapses. Neuron 61, 541-555. News and views on this paper: http://www.cell.com/neuron/abstract/S0896-6273(09)00125-1
Káradóttir, R., Hamilton, N.B., Bakiri, Y. & Attwell, D. (2008) Spiking and nonspiking classes of oligodendrocyte precursor glia in CNS white matter. Nature Neuroscience 11, 450-456. News and views on this paper: http://www.nature.com/neuro/journal/v11/n4/abs/nn0408-379.html
Schölvinck, M., Howarth, C. & Attwell, D. (2008) The cortical energy use underlying conscious perception. Neuroimage 40, 1460-1468
Peppiatt, C.M., Howarth, C., Mobbs, P. & Attwell, D. (2006) Bidirectional control of CNS capillary diameter by pericytes Nature 443, 700-704. News and Views on this paper: http://www.nature.com/nature/journal/v443/n7112/full/443642a.html
Káradóttir, R. & Attwell, D. (2006) Combining patch-clamping of cells in brain slices with immunocytochemical labelling to define cell type and developmental stage. Nature Protocols 1, 1977-1986
Káradóttir, R., Cavelier, P., Bergersen L.H.& Attwell, D (2005) NMDA receptors are expressed in oligodendrocytes and activated in ischaemia. Nature 438, 1162-1169.
Attwell, D. & Gibb, A (2005) Neuroenergetics and the kinetic design of excitatory synapses. Nature Reviews Neuroscience 6, 841-849.
Marcaggi, P. & Attwell, D. (2005) Endocannabinoid signalling depends on the spatial pattern of synapse activation. Nature Neuroscience 8, 776-781
Allen,N.J., Rossi,D.J. & Attwell,D. (2004) Sequential release of GABA by exocytosis and reversed uptake leads to neuronal swelling in simulated ischaemia of hippocampal slices. J. Neurosci. 24, 3837-3849.
Marcaggi,P., Billups,D., Attwell,D. (2003) The role of glial glutamate transporters in maintaining the independent operation of juvenile mouse cerebellar parallel fibre synapses. J. Physiol. 552, 89-107.
Hamann,M., Rossi,D., Attwell,D. (2002) Tonic and spillover inhibition control information flow through cerebellar cortex. Neuron.33, 625-633.
Attwell,D., Laughlin,S.B. (2001) An energy budget for signalling in the grey matter of the brain. J. Cereb. Blood Flow & Metab. 21, 1133-1145.
Auger,C., Attwell,D. (2000) Fast removal of synaptic glutamate by postsynaptic transporters. Neuron 28, 547-558.
Billups,D., Hanley,J.G., Orme,M., Attwell,D., Moss,S. (2000) GABAC receptor sensitivity is modulated by interaction with MAP1B. J. Neurosci. 20, 8643-8650.
Rossi,D., Oshima,T., Attwell,D. (2000) Glutamate release in severe brain ischaemia is mainly by reversed uptake. Nature 403, 316-321.
Marie,H., Attwell,D. (1999) C-terminal interactions modulate the affinity of GLAST glutamate transporters in salamander retinal glial cells. J. Physiol. 520, 393-397.
Levy,L.M., Warr,O., Attwell,D. (1998) Stoichiometry of the glial glutamate transporter GLT-1 expressed inducibly in a Chinese hamster ovary cell line selected for low endogenous Na+-dependent glutamate uptake. J. Neurosci. 18, 9620-9628.
Takahashi,M., Sarantis,M., Attwell,D. (1996) Postsynaptic glutamate uptake in rat cerebellar Purkinje neurons. J. Physiol. 497, 523-530.
Billups,B., Attwell,D. (1996) Modulation of non vesicular glutamate release by pH. Nature 379, 171 174.
Szatkowski,M., Attwell,D. (1994) Triggering and execution of neuronal death in brain ischaemia: two phases of glutamate release by different mechanisms. Trends in Neurosci. 17, 359 365.
Attwell,D., Barbour,B., Szatkowski,M. (1993) Nonvesicular release of neurotransmitter. Neuron 11, 401 407.
Sarantis,M., Ballerini,L., Miller,B., Silver,A., Edwards,M., Attwell,D. (1993) Glutamate uptake from the synaptic cleft does not shape the decay of the non NMDA component of the synaptic current. Neuron 11, 541 549.
Bouvier,M., Szatkowski,M., Amato,A., Attwell, D. (1992) The glial cell glutamate uptake carrier counter transports pH changing anions. Nature 360, 471 474
Miller,B., Sarantis,M., Traynelis,S., Attwell, D. (1992) Potentiation of NMDA receptor currents by arachidonic acid. Nature 355, 722 725
Szatkowski,M., Barbour,B., Attwell,D. (1990) Non vesicular release of glutamate from glial cells by reversed electrogenic glutamate uptake. Nature 348, 443 446
Barbour,B., Szatkowski,M., Ingledew,N., Attwell,D. (1989) Arachidonic acid induces a prolonged block of glial cell glutamate uptake. Nature 342, 918 920
Barbour,B., Brew,H., Attwell,D. (1988) Electrogenic glutamate uptake is activated by intracellular potassium Nature 335, 433 435
Sarantis,M., Everett,K., Attwell,D. (1988) A presynaptic action of glutamate at the cone output synapse. Nature 332, 451 453
Attwell,D., Borges,S., Wu,S., Wilson,M. (1987) Signal clipping by the rod output synapse. Nature 328, 522 524
Brew,H., Attwell,D. (1987) Electrogenic glutamate uptake is a major current carrier in the membrane of axolotl retinal glial cells. Nature 327, 707 709
Brew, H., Gray, P.T., Mobbs, P. & Attwell, D. (1986) Endfeet of retinal glial cells have higher densities of ion channels that mediate K+ buffering Nature 324, 466-468.
- All Publications
RPS Widget Placeholderhttps://research-reports.ucl.ac.uk/RPSDATA.SVC/pubs/DIATT18
 Close
Close















