New publication in ACS Applied Materials & Interfaces for Nguyen Lab
7 May 2020
Rapid Millifluidic Synthesis of Stable High Magnetic Moment FexCy Nanoparticles for Hyperthermia.
Magnetic nanoparticles have been receiving increased interest thanks to their potential for cancer treatment via magnetically induced hyperthermia. The most common magnetic nanoparticles studied for hyperthermia cancer treatment are iron oxide nanoparticles (IONPs). However, they possess lower magnetic moments than their metallic (non-oxidized) counterparts. Single metal nanoparticles (NPs) such as Fe, Co or FeCo alloys, have much higher magnetic moments, but are not compositionally stable, since they are sensitive to oxidation. Recently, iron carbide nanoparticles attracted significant attention for their potential use in biomedical applications. They are able to provide a trade-off between the stability against oxidation in solution, which is similar to iron oxide nanoparticles, while offering better heating efficiency.
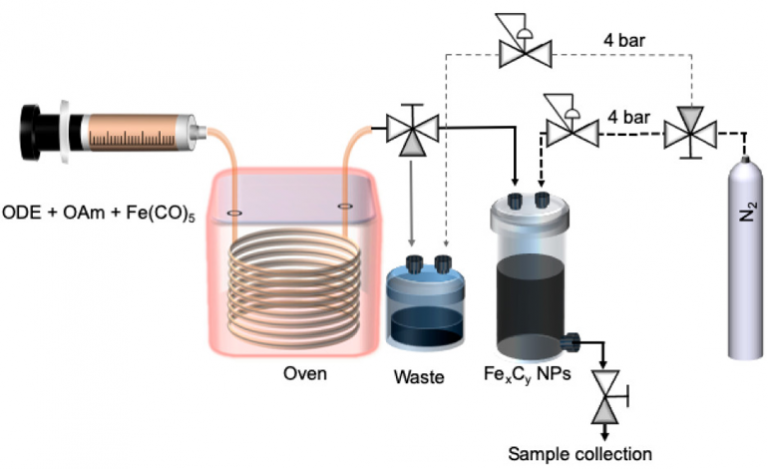
Magnetic nanoparticles (including iron carbides) are typically produced with batch chemical routes using suitable precursors in organic solvents in the presence of surfactants-stabilizing agents and with a reducing agent when needed. These synthetic routes offer somewhat limited control over the size monodispersity of nanoparticles as these reactions are sometimes hard to control with high precision and reproducibility. Thus, there is a need of better control over the synthesis conditions to achieve the desired particle properties, which we managed herein through applying a continuous flow synthetic route. Such system (see image above) allows to ensure a tight control over crucial reaction parameters such as temperature and pressure.
More specifically, in this work we synthesized monodisperse iron carbide nanoparticles in a range of sizes, which had a passivating iron oxide layer surrounding them. This layer offered prolonged chemical stability and intact magnetic properties over time. The stable magnetic behavior of these particles permitted their decent performance in hyperthermia for several months after their synthesis. Higher synthesis temperatures resulted in bigger nanoparticle sizes, which were associated with better hyperthermia efficiency. Biomedical applications do not need only a good performance of a given material, but also a prolonged duration of the material properties, which renders the material promising even for real-world scale uses. Therefore, the passivating oxide shell seems to have played an important role for this purpose.
Links
- Research paper in ACS Applied Materials & Interfaces
- Professor Thanh Nguyen’s academic profile
- UCL Physics & Astronomy
Image
- Schematic representation of the setup used for the continuous synthesis of FexCy nanoparticles including the chemical reagents involved in the reaction (ODE: 1-octadecene; OAm: oleylamine)
 Close
Close

