New publication in Physical Review Letters for Saric Lab
31 January 2020
Dispersing under pressure: clustering of membrane channels can control cell volume.
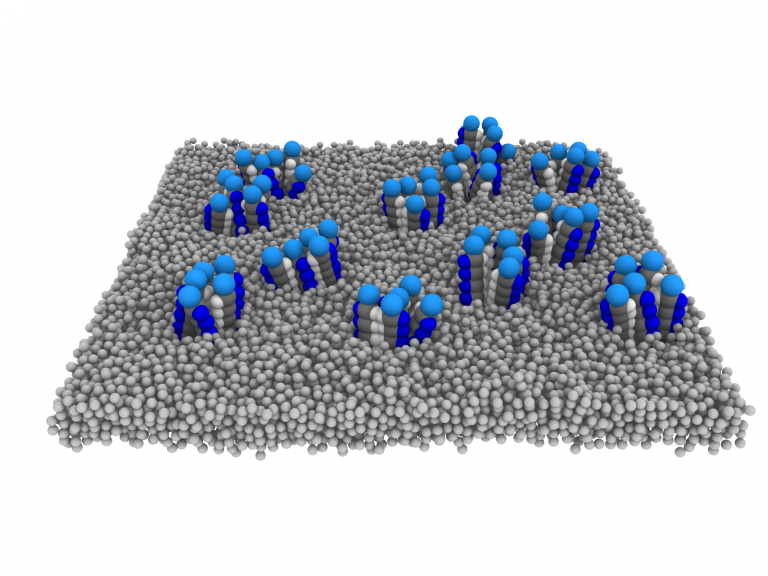
Biological molecules often separate from their environment by forming small droplets, alike droplets of oil dispersed in water. The formation of such droplets inside the living cell can regulate the cell behaviour: from changing the gene expression to altering signals and communication between the cells. In a new paper published in Physical Review Letters, IPLS student Alex Paraschiv and colleagues show that the separation of proteins into small droplets inside the cell membrane can control the volume of the whole cell, and can serve as a regulator of the cell pressure.
Cells are typically in an equilibrium with their surroundings, which includes having the same osmotic pressure as their environment, as otherwise they would shrink or explode. When the osmotic pressure of the environment suddenly changes, for instance when it rains, single cell organisms need to have a fast and efficient way of re-adjusting their internal osmotic pressure to match that of the environment. To do that, bacterial cells have evolved mechanosensitive channels – proteins that sit in the cell membrane and act as pressure valves that let molecules through to adjust the volume and pressure.
Paraschiv et al develop a minimal computer model of mechanosensitive channels inside the cell membrane to study their collective behaviour: how many channels cooperate with each other to help control the cell pressure. The authors find that by separating into small droplets, the channels help each other to quickly close when their activity is not needed. This droplet formation protects the cell from leaking. Conversely, when the osmotic pressure in the environment suddenly changes, the channel droplets quickly disperse, and individual channels efficiently open up on their own. The authors suggest that the formation of droplets serves as an additional level of the control of the cell pressure and volume.
This work is a result of a collaboration between the Saric group at UCL IPLS and Pilozota group at the University of Edinburgh.
Links:
- Research paper in Physical Review Letters
- Andela Saric's academic profile
- UCL Physics & Astronomy
- UCL MRC Laboratory for Molecular Cell Biology
- UCL Institute for the Physics of Living Systems
 Close
Close

