The Centre’s Core Management Team can also provide strategic input and high-level support for new CRUK funding initiatives, such as the Accelerator Awards and CRUK priority research funding calls.
Active CRUK Funding Opportunities
Early Detection and Diagnosis Programme Award
Funds long-term, integrated and renewable programmes of exceptional science to transform how and when early cancers and pre-cancerous states are diagnosed.
Early detection and diagnosis (ED&D) research seeks to detect and diagnose consequential precancerous changes and cancer at the earliest possible point at which an intervention might be made, reducing the burden of late-stage disease.
Funding period: Up to 5 years
Funding amount: Up to £2.5 million
Outline application deadline: 24th March 2022
Therapeutic Catalyst
The Therapeutic Catalyst will accelerate the translation of laboratory discoveries into novel cancer therapeutics.
The Therapeutic Catalyst is designed to support exploratory drug discovery efforts to validate and de-risk targets and technologies and position them for onward investment and progression. As a researcher, it means that you have the potential to take your idea from bench to patients with a single funder and partner.
The new funding opportunity is driven by our desire to improve and increase the therapeutic options available to cancer patients. The Therapeutic Catalyst will be a collaborative partnership between the researcher and Cancer Research UK (CRUK) drug discoverers, utilising the expertise of both to accelerate early therapeutic opportunities. We're in the process of changing the way we support preclinical therapeutic discovery and development and we're establishing a world-class drug discovery organisation. Further details on this will be shared soon.
Funding period: Up to 18 months
Funding amount: Up to £250k per project
Deadline: Open (until awarded)
Cancer Research UK Accelerator Award
The CRUK Accelerator Award is a Cancer Research UK initiative which provides infrastructural support to research centres in order to encourage collaboration between different organisations and boost ‘bench to bedside’ science in a particular field of cancer research or type of cancer. To date, UCL’s involvement in the Accelerator Award scheme is greater than any other CRUK Centre.
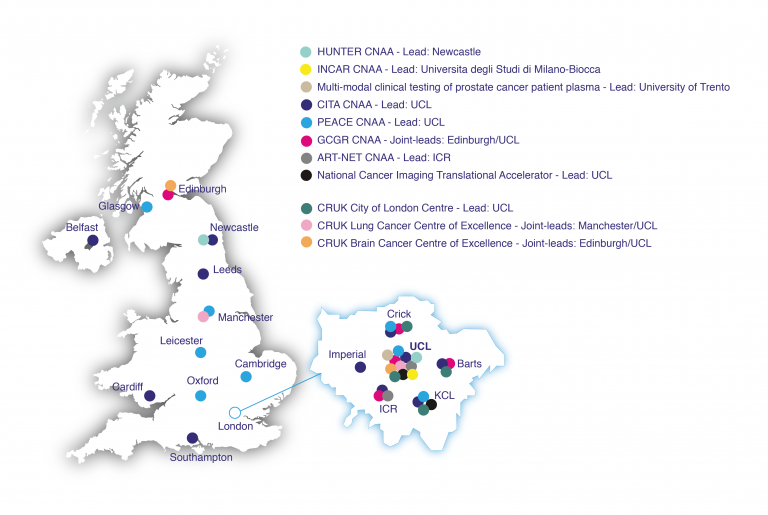
UCL CRUK Networks Collaborations and Accelerator Awards
UCL is currently leading four Accelerator Awards: the Cancer Immuno Therapy Accelerator (CITA), the Posthumous tissuE donAtion in CancEr (PEACE) study, the International Glioma Cellular Genetics Resource (GCGR), jointly led by Edinburgh and UCL and the National Cancer Imaging Translational Accelerator (NCITA). UCL are also a key member of the Advanced Radiotherapy Technologies Network (ART-NET - lead: ICR), Multi-modal clinical testing of prostate cancer patient plasma (lead: University of Trento), Hepatocellular Carcinoma Expediter Network (HUNTER - lead: Newcastle University), Innovative CAR Therapy Platforms (INCAR - lead: Università degli Studi di Milano-Bicocca) and Local Immunotherapy (LIRT - lead: Universidad de Navarra).
- National Cancer Imaging Translational Accelerator (NCITA)

Professor Shonit Punwani
UCL Lead, NCITAThe National Cancer Imaging Translational Accelerator (NCITA) is a national UK-wide medical imaging infrastructure which aims to accelerate the standardisation and translation of cancer imaging biomarkers for clinical use.
Established in 2019 with funding from a CRUK Accelerator Award of up to £10 million over 5 years, the consortium includes nine leading UK medical imaging institutions including University College London, University of Oxford, Imperial College London, King’s College London, University of Manchester, The Institute of Cancer Research, London, and The Royal Marsden NHS Foundation Trust, University of Cambridge, Newcastle University and University of Glasgow. This unique partnership provides clinical researchers across the UK with infrastructure support for multicentre clinical imaging studies including access to world-class clinical imaging facilities and expertise, quality assurance/quality support and repository data management service, including artificial intelligence (AI) tools and ongoing training opportunities.
The NCITA consortium, through engagement with NHS Trusts, pharmaceutical companies, medical imaging and nuclear medicine companies as well as funding bodies and patient groups, aims to develop a robust imaging biomarker certification process, to revolutionise the speed and accuracy of cancer diagnosis, tumour classification and patient response to treatment. The NCITA leaders work closely with the CRUK Commercial Partnerships team to ensure that new discoveries arising from NCITA’s exemplar projects and other studies supported by CRUK become available to people with cancer.
For more information visit the NCITA website and the CRUK Commercial Partnerships Imaging page. See also the NCITA Comment article published in the British Journal of Cancer.

- Multi-modal clinical testing of prostate cancer patient plasma

Professor Gerhardt Attard
UCL LeadPrecision medicine in oncology requires broadly-applicable molecular characterisation of tumours. In contrast to tissue, liquid biopsies are minimally disruptive and can be obtained repeatedly, providing real-time molecular data.
We aim to broaden the clinical utility of plasma analyses for risk stratification and treatment selection, monitoring and characterisation of resistance by increasing the amount and quality of information obtained.
The Attard Lab at the University College London Cancer Institute, in collaboration with Dr Linch and Professor Emberton, will work with Professor Demichelis at the University of Trento and a network of Italian clinical collaborators, namely Dr Ugo di Giorgi and Dr Vincenza Conteduca at the Istituto Scientifico Romagnolo per lo Studio e la Cura dei Tumori (IRST); Dr Umberto Basso at the Istituto Oncologico Veneto; Dr Orazio Caffo at the Azienda Provinciale per i Servizi Sanitari; Dr Marcello Tucci at the University of Turin, to establish a state of the art liquid biopsy test for prostate cancer patients.
Overall, our team will:
1. Implement novel computational approaches and targeted design strategies to accurately quantitate commonly recurring point mutations and copy number changes, including mono- and bi-allelic deletions at low tumour purities.
2. Integrate these genomic data with readouts of methylation status and use clinically applicable approaches for exosome isolation and analyses to obtain information on mRNA and protein expression.
3. Establish a cloud-based computational pipeline to enable standardized analysis across our network and with external collaborators.
4. Independently clinically qualify the multi-modal testing in plasma samples collected in multi-centre prostate cancer clinical trials representative of different stages in the disease spectrum.- Hepatocellular Carcinoma Expediter Network (HUNTER)
Professor Tim Meyer and Professor Mala Maini
UCL co-Leads, HUNTERPrimary liver cancer, predominantly hepatocellular carcinoma (HCC), is the second most common cause of cancer death globally. In the West, the incidence of HCC is rising and the majority of cases are detected at an advanced stage when curative interventions are no longer possible. Hence, while mortality from other cancers is falling, HCC mortality is increasing rapidly. Until recently, systemic therapy was limited to one drug which increased survival by an average of 3 months. However, recent studies using immune checkpoint inhibitors have clearly demonstrated a major benefit in up to 20% patients. The pressing scientific need is to understand how the diseased liver microenvironment and combined molecular cues determine immune cell phenotypes promoting HCC development or progression.
The HUNTER Accelerator grant from CRUK will bring together outstanding investigators from the UK, Spain and Italy who will work together to provide the foundations for new research programs in HCC. Professor Tim Meyer and Professor Mala Maini will lead the UCL contribution to the program which will define the immune microenvironment and systemic response in patients with HCC who are receiving treatment with immunotherapy. UCL will contribute to a PhD program and share resources across the partners who will focus on the development of a bioresource and improved laboratory models of disease.
It is anticipated that the outputs from this consortium will inform new therapeutic strategies and ultimately lead to improved outcomes for patients.
- Innovative CAR Therapy Platforms (INCAR)

Dr Sara Ghorashian
UCL Lead, INCARThe Innovative CAR Therapy Platform (INCAR) initiative aims to launch a novel consortium of European centres to support state of the art research into Chimeric Antigen Receptor (CAR) engineered cellular therapies. The consortium brings together world-class centres for CAR T cell therapy across Europe, and is led at UCL by Dr Sara Ghorashian. The consortium will take advantage of differences in cellular platforms, CAR design, manufacturing methods, and methods of gene transfer being investigated in studies across the programme to in turn dissect key biological factors which influence the mechanism of action of CAR-engineered cellular therapies.
At UCL we aim to define the biological characteristics which define CAR T cells with the greatest therapeutic potential as well as those with the capacity to persist long term. Dissecting the intracellular pathways which mediate these characteristics will provide a platform for their manipulation through alterations in CAR T cell manufacture or CAR engineering as well as discovery of biomarkers robustly correlated with CAR T cell fitness.The common aim of the consortium is to rapidly enhance our understanding of mechanisms responsible for biological efficacy and toxicity of CAR T cell therapy, ultimately to improve patient outcomes. We will apply a collaborative scientific approach between the leading groups in Europe, to establish standardized protocols and application of the latest research tools to ensure most the most rapid progress to our goals are achieved.
- Advanced Radiotherapy Technologies Network (ART-NET)

Professor Gary Royle
UCL Lead, ART-NETLed by Professor Kevin Harrington at the Institute of Cancer Research (ICR), a team from CRUK Centres across the UK (UCL, Leeds, Manchester and Oxford) have come together to develop, assess and implement advanced radiotherapy technologies based on stereotactic ablative radiotherapy (SABR), magnetic resonance image-guided radiotherapy (MR-Linac) and proton-beam therapy (PBT). The initiative has received £4.3 million of funding from CRUK and aims to generate and disseminate innovative national radiotherapy treatment protocols for SABR, MR-Linac and PBT.
ART-NET will initially focus on a number of disease sites, including prostate, rectal, lung and oesophageal cancers and central nervous system, but will subsequently establish mechanisms for ongoing research on these technology platforms in a range of other tumour types, providing a basis for the design and conduct of collaborative clinical trials in the UK. This will streamline the assessment of new technologies in specific tumour types to generate robust evidence on which practice change can be based. Importantly, the structure that is established by ART-NET will not only be based on sharing information across the network, but will also facilitate education and skill transfer between centres and, eventually, beyond ART-NET to the wider UK radiotherapy community.
ART-NET launched in September 2016 and has made significant progress since its inception; a cohesive, collaborative approach is firmly in place with all centres building on their strengths and disseminating their expertise across the groups. Together the working groups for each project stream are currently developing the underpinning software, methodologies and tools that will serve as the platform for really pushing forward the various areas of advanced, technical radiotherapy.
- Posthumous Evaluation of Advanced Cancer Environment (PEACE)

Dr Mariam Jamal-Hanjani
Lead-PI, PEACEThe Posthumous Evaluation of Advanced Cancer Environment (PEACE) study, led by CRUK–UCL Centre, is a multi-centre national study that aims to collect tissue and blood samples from patients with metastatic cancer. The aim of the study is to establish an unprecedented resource of samples to facilitate and encourage collaborative cancer research. In particular, the study aims to understand the process of metastatic cancer, how cancers evolve in time, and mechanisms of drug resistance. Currently there are seven study sites (UCLH, The Royal Marsden, Leicester Royal Infirmary, Christie NHS Foundation Trust, University Hospitals Birmingham NHS Foundation Trust, Guy’s and St Thomas’ and Southampton General Hospital) open, and several other sites across the UK in set up.
Doctors will invite patients with metastatic cancer - most of whom are taking part in clinical trials - to discuss with their families the issue of donating samples before deciding to be part of this pioneering research. The study allows access to cancer tissue from different sites of the body that would normally not be possible. The access to samples and clinical information from each patient will give the researchers and the scientific community a detailed timeline of the biological changes in a patient's cancer from diagnosis to death. Scientists will analyse tumour samples from the original cancer site and other organs where the cancer may have spread, along with cancer cells and DNA found in blood samples.
The PEACE study officially opened in November 2016 and since then, has made strong progress. PEACE supports a range of metastatic solid tumour types, including melanoma, lung, prostate and renal cancers, and has already recruited 100 patients. Of these 100 patients, tissue harvesting after death has been performed on 55 of them. PEACE aims to perform 500 tissue harvests in five years across different tumour types and to facilitate and address ambitious cancer research questions across different disciplines including cancer biology, tumour evolution, molecular genetics, mechanisms of drug resistance, and cancer immunology. PEACE is a £4 million investment from CRUK’s Centres’ Network Accelerator funding scheme
- Glioma Cellular Genetics Resource (GCGR)

Professor Simona Parrinello
UCL Lead, GCGRCancer Research UK have recently invested £3.4 million to support an exciting new Glioma Cellular Genetics Resource (GCGR) to accelerate research into the biology and treatment of brain cancer. Scientists at the CRUK–UCL Centre, in partnership with the CRUK-Edinburgh Centre, are building on their previous successes by reprogramming and molecular profiling of neural stem cells to generate an open collection of research tools and associated data to underpin the next era of research into glioma biology and treatments.
The research, led by Professor Steven Pollard, will generate a comprehensive collection of glioma neural stem (GNS) cell lines, normal human neural stem (NS) cell lines and patient-derived reprogrammed NS cell lines. The data from the in-depth molecular profiling of these lines will lay the foundations for future basic and translation studies into Glioma within the CRUK Centres. Importantly, these lines and associated datasets will also be made available to the wider glioma community through establishment of a web-portal/repository.
The Accelerator award is also being used to develop modified cell lines, engineered using a new CRISPR “toolbox”, to allow researchers to create useful reporters and isogenic series of engineered candidate drivers. Such patient-derived cell lines will be particularly useful in cell-based phenotypic screens for new drug development. Finally, the award aims to support and encourage young leaders in this field through its PhD training programme, which is run in conjunction with The Crick Institute.
- Cancer Immuno-Therapy Accelerator (CITA)

Professor Henning Walczak
Lead-PI, CITAThe Cancer Immuno-Therapy Accelerator (CITA) award is a £5 million Centres’ Network Accelerator Award, led by the CRUK–UCL Centre, which aims to build a collaborative network across various London CRUK research centres to advance cancer immunology and immunotherapies.
This Award funds infrastructure to enable researchers and clinicians at participating centres to track immune responses in cancer patients receiving cancer immunotherapy, investigate how and why patients stop responding to these new therapies, and develop new treatments and safe ways to give them.
Through the network created by the CITA award, scientists and doctors at UCL and UCL’s partner hospitals will be able to collaborate with researchers and clinicians within the Francis Crick Institute, King's College London, Barts, The Royal Marsden and the Institute of Cancer Research.
Dr Sergio Quezada, Scientific co-Lead on the Cancer Immuno-Therapy Accelerator Award, says: "This is a fantastic opportunity for UCL to lead a network of centres in the advancement of cancer immunotherapy. There has been very exciting progress in this field in recent years but we still need to figure out why some patients respond extremely well to these new therapies whilst others don’t, why these therapies lead to side effects in some patients and not in others and, most importantly, how to combine them in order to maximise efficacy and reduce toxicities. By collaborating across different centres in our network, we aim to answer exactly these questions. The CITA accelerator award from CRUK brings some of the world’s leading immunologists, cancer biologists and immunotherapists together from Centres across London. We hope this will foster research that will result in making cancer immunotherapies even more effective in the future.”
Professor Henning Walczak adds: “I feel very privileged to work on and build this consortium with many outstanding scientists who harbour mutual interests. I’ve worked on tumour immunology for over 20 years, so I sensed for a long time that if we were ever going to see cures in what is currently still considered incurable cancer, it would have to come from the immune system. I always thought it was the future, but now it’s here and it’s just fantastic to be part of this revolution.”
- Local Immunotherapy (LIRT)
Professor Sergio Quezada and Dr Crispin Hiley
UCL Leads, LIRT
Local Immunotherapy (LIRT) is a £3.8m CRUK Accelerator Award ley by Professor Ignacio Melero (Universidad de Navarra). This project aims to test intratumour injection of immunotherapy agents and external radiotherapy to assess efficacy and mechanisms of action. The main objective is to comparatively identify the most powerful treatment strategy, addressing the immune mechanisms of action underlying efficacy and resistance. Three clinical trials are proposed encompassing radiotherapy (SABRT or Proton beam) in conjunction with intratumoral injection of pathogen-denoting molecular patterns already undergoing clinical development of intralesional delivery as immunotherapy agents.
UCL are engaged in this project through the CRUK City of London Centre. Professor Sergio Quezada’s team will focus on T cell differentiation and dysfunction by comprehensive phenotyping of the tumour microenvironment following radioimmunotherapy. This group is part of the TRACERx consortium with Dr Crispin Hiley and Professor Charles Swanton. A team of experts lead by Dr Crispin Hiley will use clinical serial trial biopsy samples and mouse tissue specimens from experiments in Pamplona, Milan and Manchester to interrogate tumour and microenvironment evolution following local radioimmunotherapy.
 Close
Close

