Multiple Sequence Alignment (MSA) introduction
Shown below are a multiple sequence alignment of seven different representative CaMKIV across different species; their corresponding phylogenetic tree and an image of the protein's conserved domains. Note that the species were arranged in the multiple sequence alignment according to their genetic similarities determined by the phylogenetic tree. At the top is human (Homo Sapiens), followed by common chimpanzee (Pan troglodytes), which is very closely related. Then follow house mouse (Mus musculus) and common rat (Rattus novegicus), both being also mammals. These are followed by chicken (Gallus gallus), a bird, and by zebrafish (Danio rerio), a fish, both being genetically more different to mammals. Finally is thale cress (Arabidopsis thaliana), which belongs to a different kingdom. Including species which are phylogenetically very far highlights the parts of the protein which remained conserved throughtout evolution, which is a strong indicator of their importance (shown in image).
MSA Diagram
You can gain access to the relevant details of a specific domain by clicking anywhere within the corresponding coloured domain region in the diagram below.

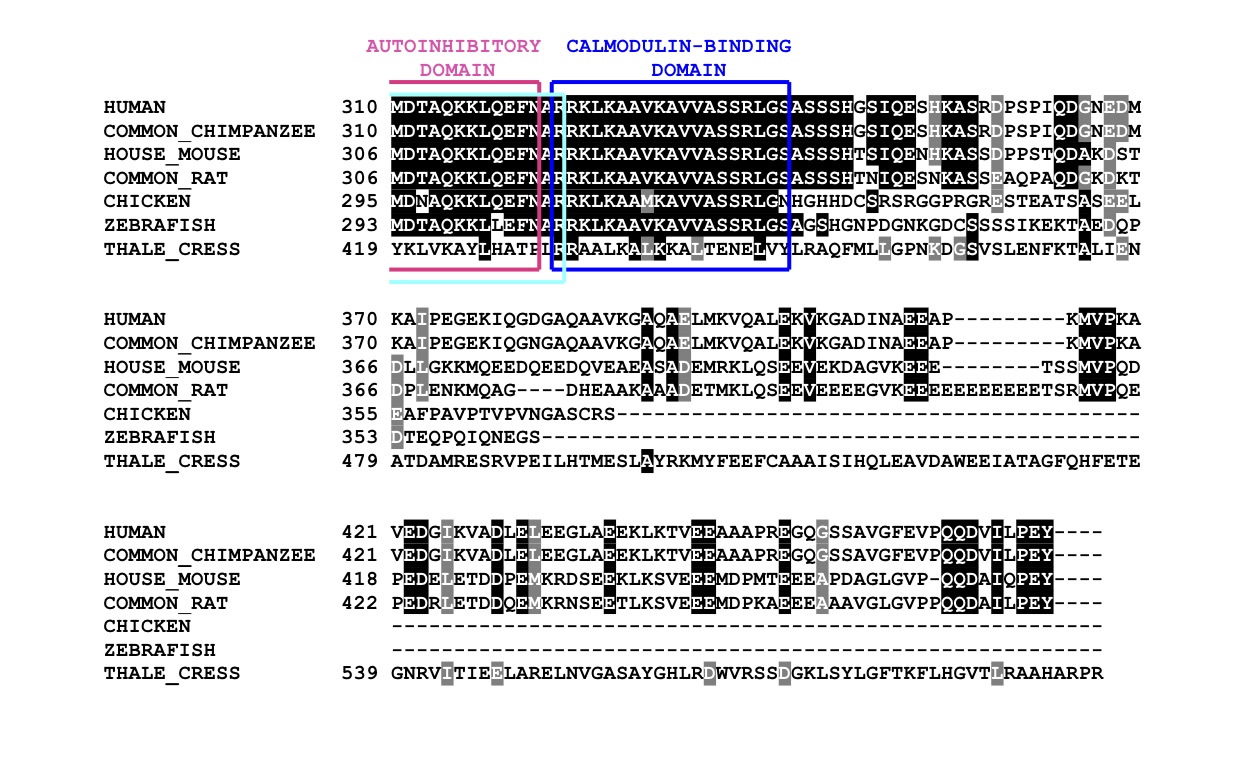
The multiple sequence alignment, produced by ClustalW, aligns different proteins according to their similarities in amino acid sequence. The outcome is colour coded and as follows: |
- Black highlight: the amino acid residue is conserved amongst all or almost all different sequences. - Grey highlight: the amino acid residue is not exactly the same as in other sequences, however, it still retains amino acid specific functions (eg: charge, shape, size...). - No highlight: the amino acid residue does not appear again in any other sequence nor are its properties similar to that of the other sequences. |
3D CaMKIV Model
Scroll over the diagram below and click on the domains to be linked to their explanations. |
%20Final.jpg)
| Shown here all four highly conserved domains of CaMKIV. The catalytic domain (yellow) makes up almost all of the protein except for the autoinhibitory helix. At the N-terminus of such helix are the autoinhibitory Domain (magenta) and the PP2A-Binding Domain (cyan), which greatly overlap (depicted as different coloured overlapping dots). By the C-terminus of the helix, and of the protein, is the Calmodulin-Binding Domain (blue). Note that the protein is a predicted model, whose sequence stops straight after the Calmodulin-Binding Domain. All four domains are exceptionally conserved amongst all the reference species. However, thale cress differs the most, having a longer sequence than the other species, leading to deletions in the alignment. |
| This protein has been predicted using the Swissmodel (an automated protein modelling server), for more information please visit: http://swissmodel.expasy.org/ |
Phylogenetic Tree (Small)
| This small phylogenetic tree was obtained with the COBALT or “constraint-based alignment tool” on NCBI and is based on protein sequence data. It is therefore not necessarily representative of the phylogenetic distance between species. |
| Despite this, the tree respects the general evolutionary relationship. This result confirms the validity of the bioinformatics tool in the case of CaMKIV. This result will become more relevant when using the tool to make the phylogenetic tree using many more species. |
| To view the larger and more comprehensive phylogenetic tree, please visist the Phylogenetic Tree section. |
| Link to COBALT ON NCBI: http://www.ncbi.nlm.nih.gov/tools/cobalt/ |
| MSA Conclusion |
| From this multiple sequnece alignment it is concluded that CaMKIV is highly conserved in evolution, mainly its four identified domains. This is a direct consequence of its importance in the T cell proliferation pathway. |

