Sleep/Wake Circuits. The transparent larval zebrafish brain makes it possible to simultaneously track the tail of the fish (to monitor behavior) while watching brain activity using a genetic encoded calcium indicator, called GCaMP. When neurons become active, they release calcium that is detected by the GCaMP molecules, which then emit light. By genetically putting the GCaMP into sleep/wake regulatory neurons, we can learn more about how these circuits coordinate to drive changes in sleep and wake behavior. We can also manipulate the activity of these neurons using techniques of optogenetics or chemogenetics, which allow us to turn the neurons ON or OFF and watch how sleep changes.
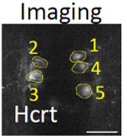
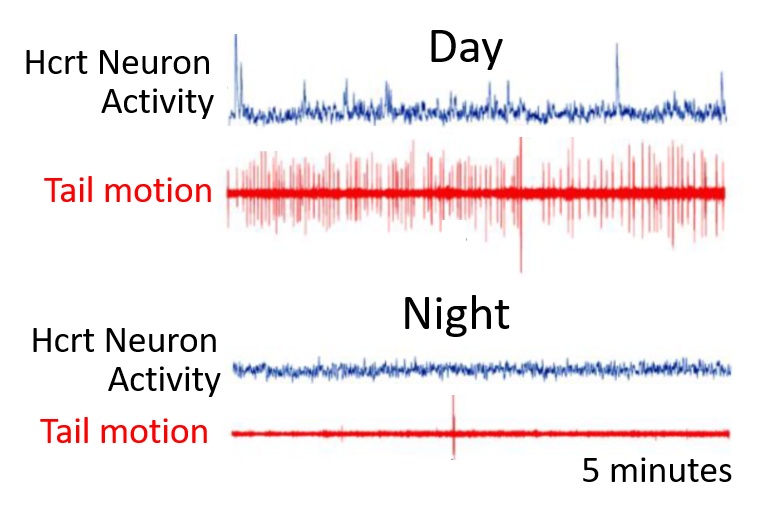 In this example, we have expressed the GCaMP in the zebrafish wake-promoting Hypocretin/Orexin neurons (Hcrt, left). These neurons are lost in human patients with the sleep disease narcolepsy. On the right, we have plotted the calcium signals (blue) from a single Hcrt neuron during the day and the night, when the larval fish is awake and asleep, respectively. The behavior of the larva is monitored by the motion of the tail (red). As observed in mammals, the fish Hcrt neurons are active during wakefulness and inactive during sleep. Other manipulations of Hcrt signaling is consistent with this peptide promoting wakefulness in fish.
In this example, we have expressed the GCaMP in the zebrafish wake-promoting Hypocretin/Orexin neurons (Hcrt, left). These neurons are lost in human patients with the sleep disease narcolepsy. On the right, we have plotted the calcium signals (blue) from a single Hcrt neuron during the day and the night, when the larval fish is awake and asleep, respectively. The behavior of the larva is monitored by the motion of the tail (red). As observed in mammals, the fish Hcrt neurons are active during wakefulness and inactive during sleep. Other manipulations of Hcrt signaling is consistent with this peptide promoting wakefulness in fish.
Sleep and Synapses. One of the most influential theory of why we sleep is the Synaptic Homeostasis Hypothesis (SHY). It states that during wakefulness, animals learn and adapt to the environment by increasing the number and strength of existing synapses, the connections between neurons. Due to the limits of resources and space, maintaining strengthened ("potentiated" in the science lingo) synapses is unsustainable. According to SHY, the function of sleep is to allow synapses to downregulate their strengths to normal, sustainable levels.
We want to observe SHY directly in the transparent larval zebrafish brain. To do this , we created a set of tools that allow us to track the location and size of excitatory and inhibitory synapses in living zebrafish larvae during sleep and wakefulness.
Sleep Homeostasis Circuits. Sleep is considered to be homeostatically regulated, which means that the duration and intensity of sleep increases after a period of deprivation. The Rihel lab uses a variety of techniques to induce a strong increase in sleep pressure, including a brief exposure to wake-promoting drugs, such as caffeine, or forced wakefulness at night. We discovered that after driving increased sleep pressure manipulations, the inhibitory neuropeptide Galanin is dramatically upregulated, and the Galanin neurons become specifically activated. Consistent with these neurons playing a sleep-homeostatic role, targeted mutations in Galanin abolishes the ability of zebrafish to respond to sleep pressure.
 This is a brain slice through the ventral part of the larval zebrafish brain. You can see the outline of the eyes to the upper and lower left. In green are areas of the brain that are upregulated during rebound sleep. The cluster of green neurons are in the preoptic area, which is full of Galanin neurons. This indicates that Galanin neurons are active during sleep.
This is a brain slice through the ventral part of the larval zebrafish brain. You can see the outline of the eyes to the upper and lower left. In green are areas of the brain that are upregulated during rebound sleep. The cluster of green neurons are in the preoptic area, which is full of Galanin neurons. This indicates that Galanin neurons are active during sleep.

 In this example, we have expressed the GCaMP in the zebrafish wake-promoting Hypocretin/Orexin neurons (Hcrt, left). These neurons are lost in human patients with the sleep disease narcolepsy. On the right, we have plotted the calcium signals (blue) from a single Hcrt neuron during the day and the night, when the larval fish is awake and asleep, respectively. The behavior of the larva is monitored by the motion of the tail (red). As observed in mammals, the fish Hcrt neurons are active during wakefulness and inactive during sleep. Other manipulations of Hcrt signaling is consistent with this peptide promoting wakefulness in fish.
In this example, we have expressed the GCaMP in the zebrafish wake-promoting Hypocretin/Orexin neurons (Hcrt, left). These neurons are lost in human patients with the sleep disease narcolepsy. On the right, we have plotted the calcium signals (blue) from a single Hcrt neuron during the day and the night, when the larval fish is awake and asleep, respectively. The behavior of the larva is monitored by the motion of the tail (red). As observed in mammals, the fish Hcrt neurons are active during wakefulness and inactive during sleep. Other manipulations of Hcrt signaling is consistent with this peptide promoting wakefulness in fish. 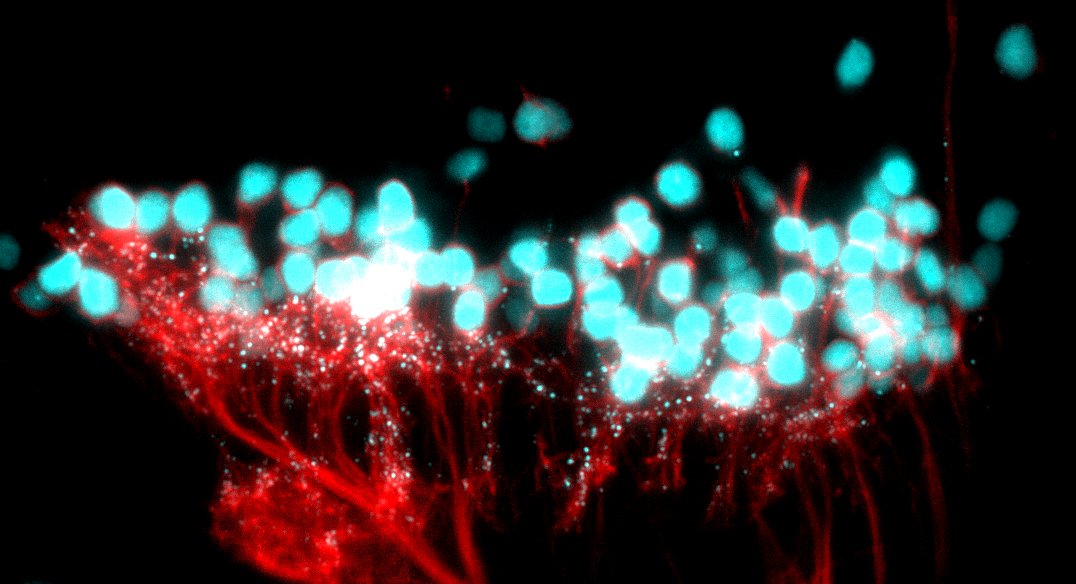
 This is a brain slice through the ventral part of the larval zebrafish brain. You can see the outline of the eyes to the upper and lower left. In green are areas of the brain that are upregulated during rebound sleep. The cluster of green neurons are in the preoptic area, which is full of Galanin neurons. This indicates that Galanin neurons are active during sleep.
This is a brain slice through the ventral part of the larval zebrafish brain. You can see the outline of the eyes to the upper and lower left. In green are areas of the brain that are upregulated during rebound sleep. The cluster of green neurons are in the preoptic area, which is full of Galanin neurons. This indicates that Galanin neurons are active during sleep.