Natalie Kirkland on her recent publication in Current Biology
In a recent publication in Current Biology, the Mao Lab and colleagues investigated how changes in tissue architecture during growth influence mitotic nuclear migration. We've asked Natalie Kirkland to tell us about the research.
What discoveries led you to your current work?
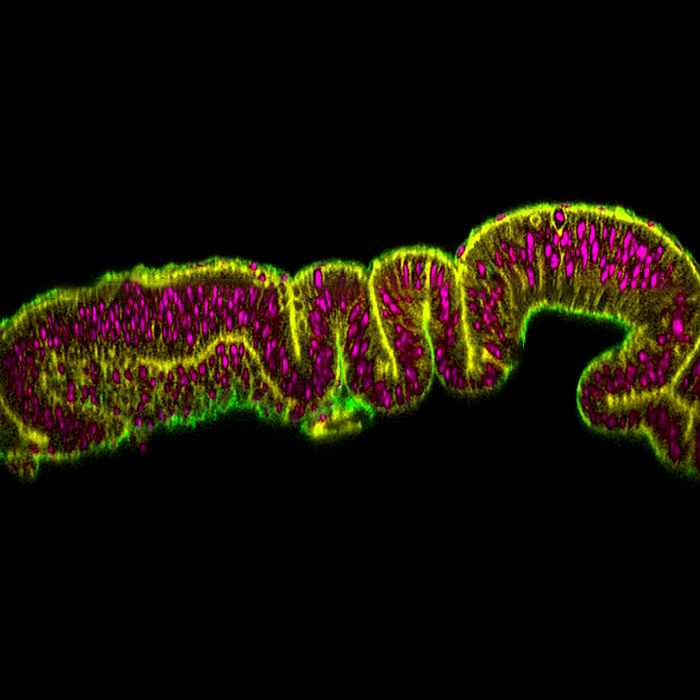
Pseudostratified epithelia are found in many developing organs as the structure allows many cells to be present in a very limited space. Cells are tightly packed together in a single layer but stagger their nuclei in rows. After rapid growth and proliferation, these cells go on to form the complex structures of our organs. However, in order to proliferate in these confined conditions, cells first translocate their nuclei to the top of the nuclear layers. At this apical region, the cells have sufficient space to “round up” during mitosis, an observation made as early as 1935 (Sauer).
Since then, many works across different species and organs have identified the cellular mechanisms that localise the nucleus to the apical surface of the epithelium. But we found it striking that the dynamics of the nuclear movement, for example, how fast and directional, could be very different depending on the species and organs studied. We were particularly inspired by two papers: the first, Leung et al, 2011, which observed in the developing zebrafish retina and neural tube that nuclei moved apically at very different speeds. The second paper, by Okamoto et al, 2014, similarly found very different nuclear dynamics when comparing the mouse and ferret brain, which differ in size and cell number. But how and why nuclear dynamics differ is only just being addressed.
What are you trying to understand?
There are clear differences in structure between the tissues that have previously been used to study nuclear movement. We therefore wanted to understand whether nuclear movement is regulated by properties outside of the cell, i.e. the tissue environment, and what aspect most strongly influences nuclear movement, e.g. the tissue height, nuclear layering, cell density, tissue curvature etc.
Research groups studying different species often found different proteins were important for nuclear movement or that movement occurred at differing times of the cell cycle. So subsequently we aimed to understand whether the mechanisms driving nuclear movement within the cell were specific to certain tissue properties.
Why is this important?
During development, our tissues change in size and shape. Cells must therefore adapt to the changing environment so that organs properly develop. For example, ensuring cell division occurs at the correct time and place is particularly important to maintain tissue integrity. It has previously been shown that when cells fail to localise the nucleus to the top of the epithelium and instead divide in the middle of the cell layer, the cell is extruded from the tissue. When many cells are displaced they can disrupt the tissue structure, which may then fail to form the adult organ.
What model system/techniques do you use?
We used the fruit fly larval wing disc, a pseudostratified epithelium that mimics the structure found in our own developing organs. During larval development the wing disc rapidly grows from a flat tear-dropped shape to a larger, folded and densely packed tissue. We took advantage of these developmental changes to directly address how tissue properties influence nuclear movement. We dissected and imaged nuclear movement in the wing disc at three time points, 24 hours apart, when there are striking differences in morphology.
In addition, we were able to genetically and physically alter tissue structure. Using a device we characterized in Duda and Kirkland et al, 2019, we stretched and compressed the tissue to acutely change nuclear-packing density within the wing disc.
What has been your most exciting discovery?
We found that as cell-packing density increases during tissue development, the speed of apical nuclear movement is reduced. But most excitingly, we also found that the increasing density influences the cell-intrinsic mechanisms required for nuclear movement. When we reduced the levels of Diaphanous, a protein that helps organize contractile apparatus at the cell membrane, the most densely-packed tissue was more greatly affected - nuclei took longer, or failed, to get to the apical surface.
Can you use an analogy to help us understand your work?
I can try! - Nuclei within the tissue can be represented by people clustering towards the stage at a concert… You’re one of them trying to get to the front. The more people, and the closer they are together, the slower you are getting there. When the concert-goers are tightly packed together you may need to push your way through to get to the front. “Pushing” is equivalent to the protein, Diaphanous - when you take it away, or don’t push, you likely don’t make it to the front!
What’s next?
It would be very interesting to understand whether the tissue properties feedback onto the cells to regulate the tissue growth via nuclear movement – it would be a new mechanism in growth control. In densely packed tissues, the slower nuclear movement may help to elongate the cell cycle and contribute to the eventual cessation of growth. The wing disc stops growing just before the larva pupate so that it can undergo morphogenesis into the adult fly wing – maybe slowing nuclear movement is involved... It would be also interesting to find out if there is a switch towards Diaphanous dependent nuclear movement as density increases? Do the cells sense compression as the tissue grows?
But that’s me done for now – I’m currently a postdoc in Prof Adam Engler’s Lab at University of California, San Diego. The many hours watching nuclei deforming as they move within the nuclear layers inspired me to investigate nuclear mechanics and their role in development and aging. I’m now using Drosophila to investigate how nuclear structural changes that occur with age can influence heart function.
I hope to come back to the questions this research has raised in the future!
Written by Natalie Kirkland
References
Sauer F. C. (1935). Mitosis in the neural tube. J. Comp. Neurol. 62, 377–405 10.1002/cne.900620207
Leung L., Klopper A. V., Grill S. W., Harris W. A., Norden C. (2011). Apical migration of nuclei during G2 is a prerequisite for all nuclear motion in zebrafish neuroepithelia. Development 138, 5003–5013.
Okamoto M., Shinoda T., Kawaue T., Nagasaka A., Miyata T. (2014). Ferret-mouse differences in interkinetic nuclear migration and cellular densification in the neocortical ventricular zone. Neurosci. Res. 86, 88–95.
Duda, M., Kirkland, N.J., Khalilgharibi, N., Tozluoglu, M., Yuen, A.C., Carpi, N., Bove, A., Piel, M., Charras, G., Baum, B., et al. (2019). Polarization of Myosin II Refines Tissue Material Properties to Buffer Mechanical Stress. Dev Cell 48, 245-260.e247.
 Close
Close