New publication in Journal of Biological Chemistry for Saiardi Lab
Phosphate is a crucially important molecule in life. The Saiardi Lab has created human cells that do not contain any of the collection of small molecules called inositol pyrophosphates. These inositol pyrophosphates were already known to regulate many cellular processes. A new publication in Journal of Biological Chemistry reveals they are also linked to cellular phosphate maintenance. In particular, inositol pyrophosphates are required for exporting phosphate out of cells. This work contributes to our understanding of cellular nutrient metabolism and control, and may support future work into metabolic diseases such as cardiovascular disease, diabetes, and cancer.
Abstract from the article:
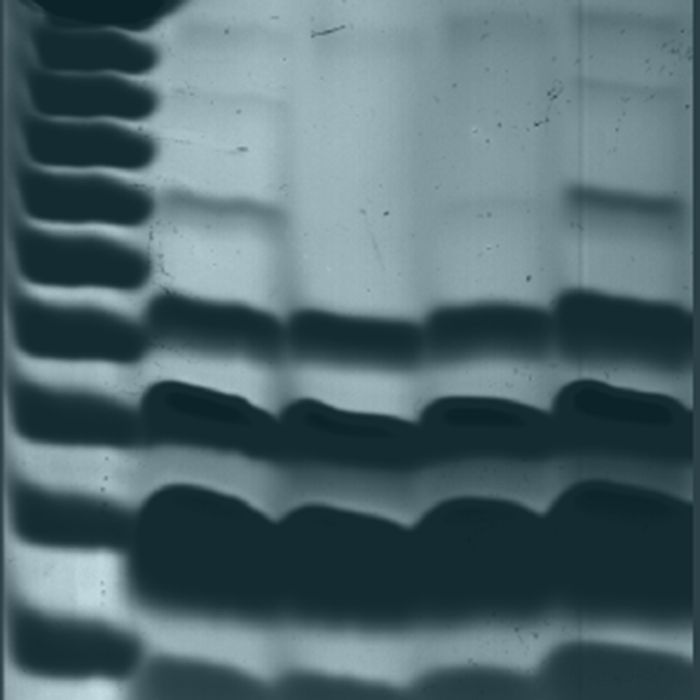
Phosphate’s central role in most biochemical reactions in a living organism requires carefully maintained phosphate homeostasis. Although phosphate homeostasis in mammals has long been studied at the organismal level, the intracellular mechanisms controlling phosphate metabolism are not well understood. Inositol pyrophosphates have emerged as important regulatory elements controlling yeast phosphate homeostasis. To verify whether inositol pyrophosphates also regulate mammalian cellular phosphate homeostasis, here we knocked out inositol hexakisphosphate kinase (IP6K) 1 and IP6K2 to generate human HCT116 cells devoid of any inositol pyrophosphates. Using PAGE and HPLC analysis, we observed that the IP6K1/2-knockout cells have non-detectable levels of the IP6-derived IP7 and IP8 and also exhibit reduced synthesis of the IP5-derived PP-IP4. Nucleotide analysis showed that the knockout cells contain increased amounts of ATP, while the Malachite green assay found elevated levels of free intracellular phosphate. Furthermore, [32Pi] pulse labeling experiments uncovered alterations in phosphate flux, with both import and export of phosphate being decreased in the knockout cells. Functional analysis of the phosphate exporter xenotropic and polytropic retrovirus receptor 1 (XPR1) revealed that it is regulated by inositol pyrophosphates, which can bind to its SPX domain. We conclude that IP6K1 and -2 together control inositol pyrophosphate metabolism and thereby physiologically regulate phosphate export and other aspects of mammalian cellular phosphate homeostasis.
 Close
Close