Visible light is focused through a specimen by a condenser lens and then it is passed through two more lenses placed at either end of a light-tight tube.
The latter two lenses each magnify the image. Limitations to what can be seen in bright field microscopy are not so much related to magnification as they are to resolution, illumination, and contrast.
Resolution can be improved using oil immersion lenses, and lighting and contrast can be dramatically improved using modifications such as dark field and phase contrast.
- Adjustment of the Interpupillary Distance
The interpupillary distance is the distance between the centres of your two pupils. The distance between the two eyepieces of the binocular microscope must correspond to your interpupillary distance. Each person has his or her own interpupillary distance and the microscope must be adjusted for your specific distance. This is true of any binocular microscope. During your first session with a new microscope you must determine the correct interpupillary distance and set the microscope for that distance. During all subsequent sessions, you must ascertain that the microscope's interpupillary distance is the correct one for you and adjust it if it is not.
If you look through the eyepieces and see two images, the interpupillary distance is not correct. To correct it, slide the eyepieces closer together or farther apart until the two fields merge to form a single circle of light. The interpupillary distance is now correct for you.
Adjustment of the Diopter Setting
The focal lengths of the two eyepieces must be adjusted to conform to differences, if any, between your two eyes so that the images in both eyepieces are in focus simultaneously. Each eyepiece is equipped with a diopter adjustment ring by which it can be focused independently of the other eyepiece.
- Adjust the interpupillary distance until it is correct for you.
- With a test slide in position on the stage, rotate the 10X ocular lens into position.
- Rotate the dipopter adjustment ring (= tube length adjustment ring) of the left eyepiece until its numerical value is the same as your interpupillary distance.
- Close your right eye and bring an object into clear sharp focus using the coarse and fine adjustment, as needed.
- Close your left eye and bring the same object into focus using the diopter ring of the right eyepiece.
Information provided by David R. Caprette of Rice University
- Basic Rules for Caring for Microscopes
- Everything on a microscope is expensive. Please be careful.
- Hold a microscope firmly by the stand, only. Never grab it by the eyepiece holder, for example.
- Hold the plug (not the cable) when unplugging the illuminator.
- Since bulbs are expensive, and have a limited life, turn the illuminator off when not in use.
- If used constantly on full power the bulb will overheat and blow (or gently melt the inside of the housing).
- Always make sure the stage and lenses are clean before putting the microscope away.
- Never use anything but good quality lens tissue on any optical surface, with appropriate lens cleaner or distilled water; organic solvents may separate or damage the lens elements or coatings.
- Cover the instrument with a dust jacket when not in use.
- Focus smoothly; don't try to speed through the focusing process or force anything.
- If it isn't working don't try to fix it unless you really know what you are doing. Make a note of the symptoms and ask someone who knows.
- Bright Field Microscopy
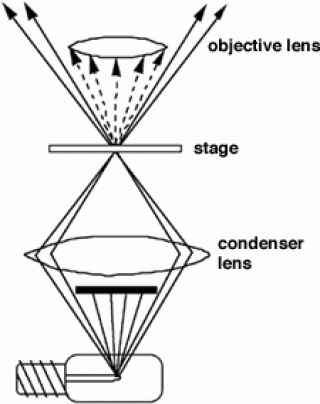
With a conventional bright field microscope, light from an incandescent source is aimed toward a lens beneath the stage called the condenser, through the specimen, through an objective lens, and to the eye through a second magnifying lens, the ocular or eyepiece. Most microscopes will have a built-in illuminator. The condenser is used to focus light on the specimen through an opening in the stage. After passing through the specimen, the light is displayed to the eye with an apparent field that is much larger than the area illuminated. The magnification of the image is simply the objective lens magnification (usually stamped on the lens body) times the ocular magnification.
Most people are usually aware of the use of coarse and fine focus knobs, used to sharpen the image of the specimen. They are frequently unaware of adjustments to the condenser that can affect resolution and contrast. Some condensers are fixed in a position others are focusable so that the quality of light can be adjusted. Usually, the best position for a focusable condenser is as close to the stage as possible. The bright field condenser usually contains an aperture diaphragm, a device that controls the diameter of the light beam coming up through the condenser so that when the diaphragm is stopped down (nearly closed) the light comes straight up through the centre of the condenser lens and contrast is high. When the diaphragm is wide open the image is brighter and the contrast is low.
Using the Bright Field Microscope
Mount the specimen on the stage
Make sure you are using the correct type of coverslip. High-magnification objective lenses can't focus through a thick glass slide; they must be brought close to the specimen, which is why cover slips are so thin. The stage may be equipped with simple clips (less expensive microscopes), or with some type of slide holder. The slide may require manual positioning, or there may be a mechanical stage that allows precise positioning without touching the slide.
Optimise the lighting
A light source should have a wide dynamic range, to provide high-intensity illumination at high magnifications, and lower intensities so that the user can view comfortably at low magnifications. Better microscopes have a built-in illuminator, and the best microscopes have control over the light intensity and shape of the light beam. If your microscope requires an external light source, make sure that the light is aimed toward the middle of the condenser. Adjust illumination so that the field is bright without hurting the eyes.
Adjust the condenser
In most cases, the condenser will already be set to its optimum position. However, there are times when you will want to check this adjustment. To adjust and align the microscope, start by reading the manual. If no manual is available, try using these guidelines. If the condenser is focusable, position it with the lens as close to the opening in the stage as you can get it. If the condenser has selectable options, set it to a bright field. Start with the aperture diaphragm stopped down (high contrast). You should see the light that comes up through the specimen change brightness as you move the aperture diaphragm lever.
Focus, locate, and centre the specimen
Start with the lowest magnification objective lens, to home in on the specimen and/or the part of the specimen you wish to examine. It is relatively easy to find and focus on samples that are fixed and stained, as with most prepared slides. However, it can be very difficult to locate living, minute specimens which move around.
Use dark field mode (if available) to find unstained specimens. If not, start with high contrast (aperture diaphragm closed down). Start with the specimen out of focus so that the stage and objective must be brought closer together. The first surface to come into focus as you bring the stage and objective together is the top of the cover slip.
If you are having trouble, focus on the edge of the coverslip or an air bubble, or something that you can readily recognize. The top edge of the cover slip comes into focus first, then the bottom, which should be in the same plane as your specimen.
Once you have found the specimen, adjust the contrast and intensity of illumination, and move the slide around until you have a good area for viewing.
Adjust eyepiece separation and focus
With a binocular microscope, you need to adjust the eyepiece separation just like you do a pair of binoculars. One or both of the eyepieces may be a telescoping eyepiece, that is, you can focus it. Since very few people have eyes that are perfectly matched, most of us need to focus on one eyepiece to match the other image. Look with the appropriate eye into the fixed eyepiece and focus with the microscope focus knob. Next, look into the adjustable eyepiece (with the other eye of course), and adjust the eyepiece, not the microscope.
Select an objective lens for viewing
The most frequently used objective lens is the 10x lens, which gives a final magnification of 100x with a 10x ocular lens. For very small objects and for details in prepared slides you will need a higher magnification. Typical high-magnification lenses are 40x and 100x. The 100x is used exclusively with oil in order to improve resolution.
Move up in magnification by steps. Each time you go to a higher power objective, re-focus and re-centre the specimen. Higher magnification lenses must be physically closer to the specimen itself, which poses the risk of jamming the objective into the specimen.
Be very cautious when focusing. Good quality sets of lenses are parfocal, that is, when you switch magnifications the specimen remains in focus or close to focused. Bigger is not always better. All specimens have three dimensions, and unless a specimen is extremely thin you will be unable to focus with a high magnification objective. The higher the magnification, the harder it is to "chase" a moving specimen.
Adjust illumination for the selected objective lens
The apparent field of an eyepiece is constant regardless of the magnification used. So it follows that when you raise magnification the area of the illuminated specimen you see is smaller. Since you are looking at a smaller area, less light reaches the eye, and the image darkens. With a low-power objective, you may have to cut down on illumination intensity. With high power, you need all the light you can get, especially with less expensive microscopes.
- Dark Field Microscopy
- To view a specimen in a dark field, an opaque disc is placed underneath the condenser lens, so that only light that is scattered by objects on the slide can reach the eye. Instead of coming up through the specimen, the light is reflected by particles on the slide. Everything is visible regardless of colour, usually bright white against a dark background. Pigmented objects are often seen in "false colors," that is, the reflected light is of a colour different than the colour of the object. Better resolution can be obtained using dark as opposed to bright field viewing.
Sophisticated equipment is not necessary to get a dark field effect, but you do need a higher intensity light since you are seeing only reflected light. At low magnification (up to 100x) any decent optical instrument can be set up so that light is reflected toward the viewer rather than passing through the object directly toward the viewer.
- Using a dissecting microscope
- To set up a dissecting microscope for "dark field" viewing, the specimen should be placed over an opening so that light reflects only from surfaces between the cover slip and slide, not from a surface beneath the slide. You may need to make a stand to hold the slide. The surface beneath the opening should be flat black. Turn off any built-in illuminator. Aim a high-intensity light source toward the specimen at an angle, from the top or side through a glass dish or jar.
- Using a high powered microscope
- With a compound microscope, dark field is obtained by placing an occulting disk in the light path between the source and the condenser. A cheap set of occulting disks can be prepared by cutting circular pieces of black electrical tape ranging from about the size of a five-pence piece up to a diameter that equals the width of the slide, and sticking them to the slide in a row. The circles should be spaced well apart. A specimen is placed on the microscope stage as usual, and the illumination should be made as uniformly as possible. If there is an aperture diaphragm in the condenser (contrast lever), it should be opened up wide. After focusing at low power, the slide with occulting disks is placed in the light path between the source and the condenser, bringing it as close to the bottom of the condenser as it will go.
Start with the largest disk, sliding it around until it is directly in the centre of the light path. Increasing the illumination should then produce a good dark field effect. To optimize, first try stopping down the field diaphragm to get the best contrast between the background and specimen. Try to match the size of the occulting disk to the field diameter, so that the edge of the disk is just outside the field of view - smaller disks are appropriate for higher power objectives. Vertically, the disk should be a close to the condenser as possible, to make the contrast the greatest. On microscopes with built-in dark field equipment, the view is so impressive because the occulting disk is built into the condenser - very close and focused. After testing the set-up this way, a stand might be rigged to fit under the microscope, so the slide can be placed in position without holding it. Something that 'grabs' the condenser and supports the occulting disks would be ideal. The less you have to mess with, the better.
Suspensions of cells and samples of pond water look spectacular in dark field. While specimens may look washed out and lack detail in bright field, protists, metazoans, cell suspensions, algae, and other microscopic organisms are clearly distinguished and their details show up well. At 100x you can readily see bacteria, and even distinguish some structures (rods, curved rods, spirals, or cocci) and movement. Non-motile bacteria look like vibrating bright dots against a dark background. Motile bacteria can be seen moving in a definite direction, sometimes remarkably fast. In pond water samples you may find Spirillum volutans, a very large (up to 0.5 mm) motile spiral bacterium.
- When to use dark field illumination
Dark field illumination is most readily set up at low magnifications (up to 100x), although it can be used with any dry objective lens. Any time you wish to view everything in a liquid sample, debris and all, dark field is best. Even tiny dust particles are obvious. Dark field is especially useful for finding cells in suspension. Dark field makes it easy to obtain the correct focal plane at low magnification for small, low-contrast specimens.
- Phase Contrast
Most of the detail of living cells is undetectable in bright field microscopy because there is too little contrast between structures with similar transparency and no colour. Unless the specimen mount is extremely thin, dark field mode may distort details.
Highly refractive structures bend light to a much greater angle than do structures of low refractive index. The same properties that cause the light to bend also delay the passage of light by a quarter of a wavelength or so. In a light microscope in bright field mode, light from highly refractive structures bends farther away from the centre of the lens than light from less refractive structures and arrives about a quarter of a wavelength out of phase.
Light from most objects passes through the centre of the lens as well as to the periphery. If the light from an object to the edges of the objective lens is retarded by a half wavelength and the light to the centre is not retarded at all, then the light rays are out of phase by a half wavelength. They cancel each other when the objective lens brings the image into focus. A reduction in the brightness of the object is observed. The degree of reduction in brightness depends on the refractive index of the object.
Phase contrast is preferable to bright field microscopy when high magnifications (400x, 1000x) are needed and the specimen is colourless or the details are so fine that colour does not show up well.
In phase contrast, a phase plate is placed in the light path. Barely refracted light passes through the centre of the plate and is not retarded. Highly refracted light passes through the plate farther from the centre and is held back another one-quarter wavelength.
Using Phase Contrast
Phase contrast condensers and objective lenses add considerable cost to a microscope. Most of the microscopes available in our microscope rooms have phase - the settings on these should not be changed unless you really know what you are doing!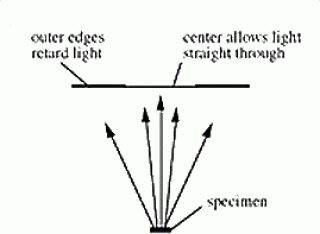
To use phase contrast the light path must be aligned. An element in the condenser is aligned with an element in a specialized phase contrast lens. This usually involves sliding a component (a phase telescope) into the light path. The elements are either lined up in a fixed position or are adjusted by the observer until the phase effect is optimized. If in doubt please ask someone to do this for you.
Generally, more light is needed for phase contrast than for corresponding bright field viewing, since the technique is based on a diminishment of the brightness of most objects.
There are some limitations of phase contrast microscopy:
- The images are usually surrounded by "halos" around the outlines of details. These are optical artefacts, which may obscure the boundaries of details.
- There may be a reduction in the image resolution.
- Phase contrast does not work well with thick specimens because shifts in phase occur from areas slightly below or slightly above the plane that is in focus. Such phase shifts confuse the image and distort image detail.
- Microscopy with Oil Immersion
When light passes from a material of one refractive index to a material of another, such as from glass to air or from air to glass, it bends. Light of different wavelengths bends at different angles, so that as objects are magnified the images become less and less distinct. This loss of resolution becomes very apparent at magnifications above 400x or so. In fact, as you will see later, even at 400x the images of very small objects are badly distorted.
Placing a drop of oil with the same refractive index as glass between the cover slip and objective lens eliminates two refractive surfaces and considerably enhances resolution so that magnifications of 1000x or greater can be achieved.
To use an oil immersion lens, first, focus on the area of the specimen to be observed with the high dry (400x) lens. Place a drop of immersion oil on the cover slip over that area, and very carefully swing the oil immersion lens into place. Focus carefully, preferably by observing the lens itself while bringing it as close to the cover slip as possible, then focusing by moving the lens away from the specimen. When in focus the lens nearly touches the cover slip. The focal plane is so narrow that it is very easy to focus right past it. If you are focusing on the specimen, you can drive the lens right into it.
When to use oil immersion lenses
Use an oil immersion lens when you have a fixed (dead - not moving) specimen that is no thicker than a few micrometres. Even then, use it only when the structures you wish to view are quite small - one or two micrometres in dimension. Oil immersion is essential for viewing individual bacteria or details of the striations of skeletal muscle. It is nearly impossible to view living, motile protists at a magnification of 1000x, except for the very smallest and slowest.
A disadvantage of oil immersion viewing is that the oil must stay in contact, and the oil is viscous. A wet mount must be very secure to use oil. Oil immersion lenses are used only with oil, and oil can't be used with dry lenses, such as your 400x lens. Lenses of high magnification must be brought very close to the specimen to focus and the focal plane is very shallow, so focusing can be difficult. Oil distorts images seen with dry lenses, so once you place oil on a slide it must be cleaned off thoroughly before using the high-dry lens again. Oil on non-oil lenses will distort viewing and possibly damage the coatings.
 Close
Close

