Network engagement & project development
Multidisciplinary project teams will comprise academic researchers from across the EMINENT network, working with GSK lead scientists, focussed on delivering novel experimental studies with assets from the GSK clinic ready portfolio- Details of the latest GSK pipeline.
Following formation of a project team around a specific novel focus, new projects will be worked up through a detailed scientific oversight process to ensure the delivery of novel science which aligns with the overall aims of EMINENT. Details of project scope and network oversight are outlined in the boxes below.
Investigators interested in engaging with EMINENT should contact their Institutional EMINENT PI (see Key People) for their institution or alternatively contact the EMINENT Programme Manager for more information.
Further information
- Project Scope
Projects will be focussed on utilising unlicensed clinic ready GSK assets to enable innovative experimental medicine studies with the ultimate aim of delivering novel therapeutic options for patients in areas of high unmet medical need. The scope of project activity will range from research aimed at establishing pre-clinical and clinical evidence in support of novel targets, defining pathways and disease mechanisms, and generating a robust tool kit (e.g. novel biomarkers, imaging modalities) to deliver experimental medicine studies in humans.
All proposals should incorporate the following elements
- Justification for the research in terms of advancing mechanistic disease understanding, novel biomarker discovery or efficacious therapeutic intervention, referencing in particular why the proposed research uniquely aligns with EMINENT
- Clear line of sight to an experimental medicine study- either included within programmatic awards or providing groundwork for EM studies through a starter project award
- Clear focus on using unlicensed GSK Clinic Ready Assets. Details on the current GSK pipeline. Applicants must demonstrate they have internal GSK support for project proposals, with the implicit proviso that proposed use of the asset must be outside the current focus of the GSK portfolio
- Evidence of matched MRC and GSK (in-kind) funding-justification must be provided if cost matching is unachievable
- A clearly mapped milestone-driven approach with go/ no-go decision points for project progression
- Clear potential to collaboratively utilise expertise and tools across the EMINENT network
- Clear opportunity for the exchange of knowledge, training and skills between academic partners and GSK
- Strategies for prompt publication of data
- Project Streams
EMINENT project applications should fall into one of two broad streams:
Starter projects:
- Exploratory projects delivering early target or platform development, with line of sight to clinical study enabling
- £75-150K
- 6-12 month duration
- Single or multicentre
Programmatic pathway projects:
- Aiming to deliver clinical studies with GSK assets to evaluate novel biomarkers, technologies, Proof of Mechanism or efficacy
- >£1M
- 4-5 year duration
- Multicentre
- Scientific Review
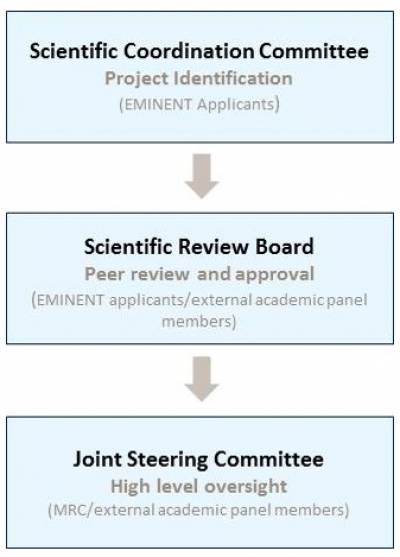
Scientific Coordination Committee Approval (approx 3 weeks)
Once approval has been sought at institutional PI level, a one page Project Outline proforma will be submitted the the Scientific Coordination Committee (SCC) detailing
- Scientific rationale for the project
- Alignment with EMINENT scope (as outlined in EMINENT scope)
- Alignment with EMINENT research priorities
Project Outline submission deadlines
It should be noted that in order for EMINENT to devliver maximal impact, the portfolio of projects in the EMINENT consortium is actively managed by the EMINENT SCC. It is critical that applicants actively engage with their institutional PIs at an early stage before submitting Project Outlines in order to establish project scope and the current priorities of the EMINENT network.
Should projects be supported in principle by the SCC, applicants will be invited to develop a full application for submission by the Scientific Review Board
Scientific Review Board Approval (approx 10-12 weeks)
Applicants will be invited to develop a detailed application, building on the Project Outline proforma and detailing:
- Project timelines and deliverables
- Project milestone objectives
- Approved project costings (from both the academic and GSK teams)
- Next steps on study completion
Full application submission deadlines
This will be reviewed in detail by the EMINENT Scientific Review Board comprising members of the Scientific Coordination Committee and MRC approved external academic members.
Applicants will be given feedback from the SRB, with potential outcomes of:
- Award
- Award in principle
- Major revisions
- Decline
For outcomes 2 and 3 in principle funding support will be predicated on applicant responses to feedback
EMINENT Joint Steering Committee (approx 1-2 weeks)
Approval for support will be sought from the EMINENT JSC on decisions made by the EMINENT SRB to trigger release of support.
- Post Award
Projects will progress through a series of clearly defined and carefully monitored milestones and go / no-go decision points; enabling active management of the portfolio of projects within EMINENT, to ensure the delivery of translational impact from the EMINENT network.
 Close
Close