Study Overview
Study I: CROMIS-2 (AF)
Prospective cohort study of patients anticoagulated after cardioembolic stroke
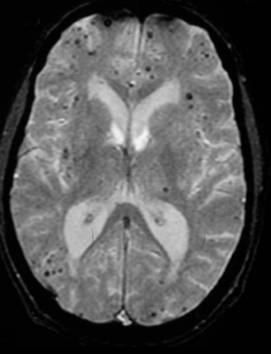
Study II: CROMIS-2 (ICH)
Observational and genetics study of intracerebral haemorrhage
We will also recruit 600 patients admitted to participating centres with ICH (with a target of at least 300 anticoagulant-related ICH cases) and collect DNA to increase the power of the genetic studies. We will collect clinical and imaging data from these ICH cases to investigate risk factors associated with anticoagulant-related ICH compared to non anticoagulant-related ICH.
The Research Questions
Primary Questions
- Does the presence of CMBs help predict the risk of symptomatic oral anticoagulant-related ICH in patients who are anticoagulated following cardioembolic stroke due to non-valvular AF?
- Do the burden (number) and distribution of CMBs at baseline influence the risk of ICH in this cohort?
Secondary Questions
- In patients anticoagulated after ischaemic stroke due to non-valvular AF, are CMBs associated with an increased risk of recurrent TIA, ischaemic stroke or death?
- Are genetic polymorphisms related to the integrity of brain small vessels or anticoagulant metabolism associated with an increased risk of ICH?
- Are CMBs a better predictor of oral anticoagulant-related ICH than clinical risk factors and/or leukoaraiosis on MRI scans?
- Can a useful risk prediction model incorporating clinical, imaging and genetic factors be developed to assess the risk of best practice oral anticoagulant-related ICH?
- Can we identify new genetic, clinical or radiological risk factors associated with anticoagulant-related ICH?
The Hypothesis
Because oral anticoagulant associated ICH is associated with increased age and previous stroke, and often occurs with anticoagulation intensity within the therapeutic range[1], it is likely that the mechanism underlying the high risk is related to individual patient factors, for example an age-related disorder of small brain blood vessels, such as cerebral amyloid angiopathy (CAA) or hypertensive small vessel disease. In keeping with this hypothesis, some studies suggest that leukoaraiosis - a confluent deep white matter abnormality seen as low attenuation on computed tomography (CT) or high signal on T2-weighted MRI, which is a marker of small vessel disease - increases the risk of oral anticoagulant-related ICH.2,3 CMBs are associated with leukoaraiosis: however, because they provide direct evidence of leakage of blood from pathologically fragile small vessels, we hypothesize that microbleeds are a better predictor of oral anticoagulant-associated ICH than leukoaraiosis alone.
Methods
 Study I (AF)
Study I (AF)

Patients will have genetic testing and standardized MRI including GRE at baseline, with follow-up at 3,6,12 and 18 months by postal questionnaire (and clinical assessment or medical records surveillance after suspected events), and final clinical assessment at 2 years.
Study II (ICH)
Patients will a standardized MRI including GRE at baseline (or CT scan), with follow-up at 6 months by postal questionnaire (and clinical assessment or medical records surveillance after suspected events).
Inclusion and exclusion criteria
Study I: CROMIS-2 (AF)

- Adult (≥18y; no upper limit) patients with a clinical diagnosis of non-valvular AF (verified by ECG) with intention to treat with best practice oral anticoagulants (e.g. warfarin)
- Previous ischaemic stroke or TIA diagnosed by treating clinician
- All patients must be able to have GRE MRI before (or within 1 week) of starting best practice oral anticoagulant
Exclusion criteria:
- Any MRI contraindications
- Previous use of oral anticoagulation
- Definite contra-indication to oral anticoagulation
- Serious head injury (resulting to loss of consciousness)
Study II: CROMIS-2 (ICH)
Inclusion criteria:
- Adult (≥18y) patients treated at participating centres with confirmed ICH (confirmed on CT or MRI scans) with or without a history of anticoagulant use at the time of the ICH
Exclusion criteria:
- Known underlying structural cause for ICH, e.g arteriovenous malformation, tumour, cavernoma, intracranial aneurysm, haemorrhagic transformation of an infarct
- Major head trauma (causing loss of consciousness and thought to be sufficient to have caused the ICH) in previous 24 hours.
Expected outcomes
A successful predictive model for ICH risk after best practice oral anticoagulation for AF will help to determine whether genetic or CMB screening should be used in clinical practice and future trials. New genetic, clinical and radiological risk factors associated with anticoagulant-related ICH will be identified.
Planned recruitment period
Original recruitment was planned to run from July 2011 and will continue for 36 months. An extension was granted to continue recruiting more patients until July 2015 (48 months in total).
Funding
The study has been funded by The Stroke Association and The British Heart Foundation for 5 years (to include patient follow up).


References
- Rosand J, Hylek EM, O'Donnell HC, Greenberg SM. Warfarin-associated hemorrhage and cerebral amyloid angiopathy: a genetic and pathologic study. Neurology. 2000;55:947-51
- Smith EE, Rosand J, Knudsen KA, Hylek EM, Greenberg SM. Leukoaraiosis is associated with warfarin-related hemorrhage following ischemic stroke. Neurology. 2002;59:193-7
- Gorter JW. Major bleeding during anticoagulation after cerebral ischemia: patterns and risk factors. Stroke Prevention In Reversible Ischemia Trial (SPIRIT). Neurology. 1999;53:1319-27
 Close
Close

