Gut Development and Repair Group
determination in both normal and pathophysiological conditions. We have a particular focus on investigating the underlying molecular and genetic mechanisms of gut disease to improve diagnosis and develop novel therapeutic approaches, including stem cells and gut tissue engineering, for their treatment.
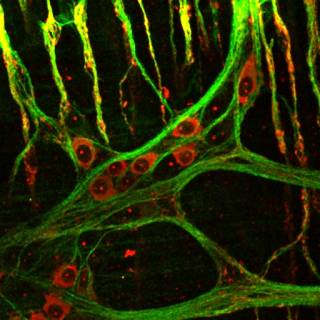
Normal gastrointestinal (GI) function requires the coordinated interaction of the enteric neurons and glial cells that comprise the enteric nervous system (ENS), interstitial cells of Cajal, and smooth muscle cells. Defects in the development of these cell types results in a range of commonly occurring gut disorders/diseases including Hirschsprung disease (aganglionic megacolon), intestinal pseudo-obstruction, and other motility defects.
Understanding how the gut develops and how molecular and cellular pathogenesis lead to neuromuscular diseases drives our research in developing regenerative medicine approaches to better treat gut disorders.
 |  |
We take a number of approaches, in collaboration with groups locally, nationally and internationally to:
- Investigate the mechanisms underlying enteric nervous system (ENS) and interstitial cells of Cajal (ICC) development
- Explore how coordinated activity in the gut develops
- Better understand the underlying pathophysiology of gut motility disorders
- Develop novel stem cell-based therapies for gut disorders such as Hirschsprung disease
- Use tissue engineering approaches to manufacture replacements for diseased gut
- Group Members
Dr Ben Jevans
Research Fellow
benjamin.jevans.13@ucl.ac.uk
UCL Profiles page: Ben Jevans- Projects
Developing a human pluripotent stem cell-based strategy for treating Hirschsprung disease
Together with Dr. Anestis Tsakiridis and Prof. Peter Andrews (University of Sheffield), we are currently establishing the preclinical basis for regenerative medicine approaches for the treatment of Hirschsprung disease.
Hirschsprung disease is a life-threatening intestinal disorder caused by an absence of intrinsic nerve cells (aganglionosis) in the most distal GI tract. It occurs in approximately 1 in 5000 live births, making it one of the most common congenital diseases affecting the gut. Given that intrinsic gut nerve cells (enteric neurons) mediate the contractions necessary for normal gut function, their absence in Hirschsprung patients causes severe constipation or intestinal obstruction. The only treatment available is surgical removal of the affected part of the bowel combined with a 'pull through' procedure, which entails connecting the healthy part of the gut to the anus. However, the surgery necessitates retention of part of the abnormal gut including the anal sphincter, which is likely to account in part for the long-term, often life-long, gastrointestinal problems and poor quality of life suffered by the majority of patients. Surgery, readmission and outpatient hospital appointments which are required for the management of this condition present a significant burden for the healthcare system.
Recent advances in the understanding of development of the gut's intrinsic (enteric) nervous system and pathogenesis of the disease, as well as considerable progress in regenerative medicine, have highlighted potential for alternative treatments, such as cell replacement therapy. Although preclinical testing has demonstrated that cell therapy should be a viable option for treating Hirschsprung disease, the availability of human enteric neurons from post-natal gut remains a bottleneck for the development of cell-based therapies. An attractive alternative source is offered by pluripotent stem cells, where there is significant potential to generate appropriate cells for therapy, both in terms of numbers and the ability to tailor cell properties. We have recently developed efficient protocols to generate human neural crest cells and enteric nervous system progenitors from human pluripotent stem cells. In this project, we will test the ability of these progenitors to correct for the lack of enteric neurons in models of Hirschsprung disease following transplantation. We will also optimise the current methods of generating and purifying human enteric nervous system progenitors, from pluripotent stem cells, and will develop new transplantation protocols and models of Hirschsprung disease.
Using ex vivo organotypic cultures to investigate donor cell integration in the gut
- Teaching
I currently co-lead the Molecular Aspects of Cell and Gene Therapy module, at UCL, which forms a core part of the UCL Great Ormond Street Institute of Child Health MSc. Cell and Gene Therapy
For more information on the Programme please click here.
- Funders, Collaborators and Partners








 Close
Close