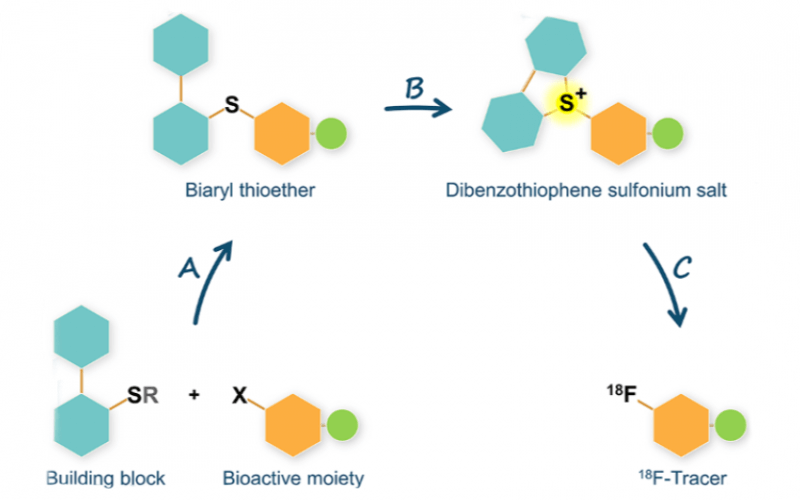
Radiochemistry

UCL Good Manufacturing Practice (GMP) Facility
The UCL Good Manufacturing Practice (GMP) Facility is a state-of-the-art academic radiopharmaceutical unit that produces injectable diagnostic agents for imaging in man.
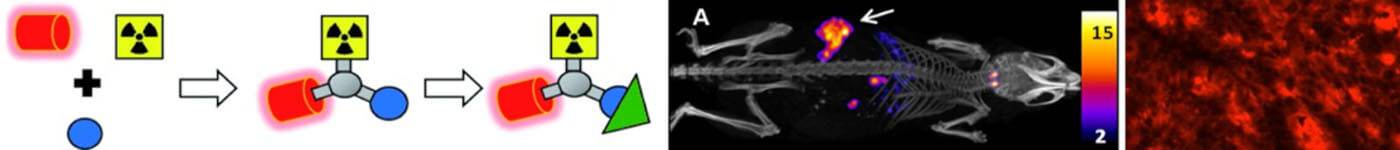
Discriminatory ability of next-generation tau PET tracers for Alzheimer’s disease
The high variability of cortical tracer binding within the Alzheimer’s disease group suggests all compounds are suited to differentiate Alzheimer’s disease from other dementias.

Imaging tau pathology in Alzheimer’s disease with positron emission tomography
Imaging tau pathology in Alzheimer’s disease with positron emission tomography: lessons learned from imaging-neuropathology validation studies.
Our work
Our experts




Dr Fatih Sirindil


Frazer Twyman


Dr Dylan Pritchard
- Research Fellows
- Blanca Szulc
- Steven Yap
- Agostinho Lemos
- Thibault Gendron
- Raul Pereira
- Chris Blunt
- Laure Benhamou
- Eva Galante
- Ran Yan
- Carlos Perez-Medina
- PhD students
- Melissa Wren
- Brian Pak Kwan Sin
- Klaudia Cybulska
- Elena Yiannaki
- Niral Patel
- Staff
- Camila Marcolan


Selected Publications
- Moscoso A, Wren MC, Arstad E, et al. (2022). Imaging tau pathology in Alzheimer's disease with positron emission tomography: lessons learned from imaging-neuropathology validation studies. Molecular Neurodegeneration, 17 (1).
- Sirindil F, Maher S, Sander K, Årstad E, et al. (2022). Oxidation-Cyclisation of Biphenyl Thioethers to Dibenzothiophenium Salts for Ultrarapid 18F-Labelling of PET Tracers. International Journal of Molecular Sciences, 23 (24).
- Sirindil, F., Arstad, E., Sander, K., Awais, R., Twyman, F., Marcolan, C., Glaser, M. (2022). P-161 - Automated production of [(18)^F]AldoView: first translation of a sulfonium salt precursor to GMP.
- Yap S, Arstad E, Sander K, et al. (2021). Ability Of Next-Generation Tau Pet Tracers To Discriminate Alzheimer’s Disease Histopathology From Other Dementias.
- Sander K, Gendron T, Arstad E, et al. (2021). Development of [¹⁸F]AldoView as the First Highly Selective Aldosterone Synthase PET Tracer for Imaging of Primary Hyperaldosteronism. Journal of Medicinal Chemistry.
- Yap S, Årstad E, Sander K, et al. (2021). Discriminatory ability of next-generation tau PET tracers for Alzheimer's disease. Brain.
- Bofinger R, Glaser M, Sander K, et al. (2021). Drug delivery, biodistribution and anti-EGFR activity: theragnostic nanoparticles for simultaneous in vivo delivery of tyrosine kinase inhibitors and kinase activity biosensors. Nanoscale.
- Vicente-Rodríguez M, Awais R, Sander K, et al. (2021). Resolving the cellular specificity of TSPO imaging in a rat model of peripherally-induced neuroinflammation. Brain, Behavior, and Immunity.
- Marcolan C, Glaser M, Twyman F, Sander, K, Awais R, Arstad E. (2020). A Generic GC Protocol for the Residual Solvent Analysis of PET Radiopharmaceuticals.
- Yap S, Arstad E, Sander K, et al. (2020). High sensitivity of tau radiotracers to depict tau spread in prodromal cases of Alzheimer's disease.
- Gendron T, Pereira R, Årstad E, et al. (2020). Iron(II)/Persulfate Mediated Newman-Kwart Rearrangement. Organic Letters.
Facilities
Our labs include a radiochemistry research lab and the UCL Good Manufacturing Practice (GMP) Facility in the Kathleen Lonsdale Building, created with generous funding from HEFCE and the UCL Hospitals Biomedical Research Centre. This helps the research and production teams to work towards truly translational research.
Radiochemistry research lab
The radiochemistry research lab is designed to allow radiochemistry method development, automated radiotracer production for preclinical studies, and translational nuclear imaging in human specimens. Three shielded fume hoods and two research hot cells equipped with Scintomics synthesis modules are complemented by a dedicated quality control lab.





UCL GMP Facility
Custom-designed to produce investigational diagnostic agents for imaging in man, the UCL GMP Facility specialises in the manufacturing of short-lived radiotracers as well as tracers for cutting-edge imaging techniques such as hyperpolarised MRI. The facility houses a class C cleanroom with four Tema hot cells and two class A isolators. Tracer production is carried out on the Trasis AllinOne platform. The dedicated quality control lab features Agilent and Shimadzu high-performance liquid chromatography systems, integrated with Lablogic radio detectors, an Agilent gas chromatography, radio TLC system and Endosafe equipment for pyrogen testing.

Interested in joining us?
Collaborations are strongly encouraged both within and external to UCL. We provide radiotracers and services for clinical and preclinical projects through the UCL GMP Facility and the research facility, respectively. We constantly implement new tracer products as well as radiochemical methods and imaging techniques. Please do get in touch if you would like to work with us.
Contact details
- Prof. Erik Årstad | Director (e.arstad@ucl.ac.uk)
- Dr Kerstin Sander | PET Pharmacology Group Lead (k.sander@ucl.ac.uk)
- Dr Ramla O. Awais | GMP Manager (r.awais@ucl.ac.uk)
Postal and Visiting Address
Centre for Radiopharmaceutical Chemistry (CRC)
Kathleen Lonsdale Building
5 Gower Place
London, WC1E 6BS
 Close
Close