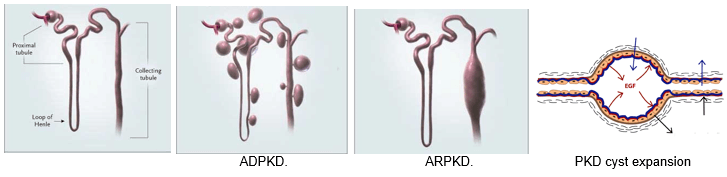
Experimental Nephrology

Can you support greater patient involvement in planning renal research?
Professor Alan Salama explains some research plans at the Royal Free Kidney unit to get greater patient involvement into planning research and organising research priorities.

Image-based machine learning platforms to diagnose urinary tract infection (UTI)
In 2022, Harry Horsley explained his use of active learning and a pixel classifier (random forest algorithm) for a system with the potential to revolutionise diagnosis and patient management.
Our work
Our experts

Prof. Jill Norman (Head)

Prof. Ben Caplin (Deputy Head)


Dr Sally Hamour




Dr Xiong Zhong Ruan



Dr John Connolly


Selected Publications
- International Society of Nephrology's International Consortium of Collaborators on Chronic Kidney Disease of Unknown Etiology (2023). Challenges and opportunities in interventions for chronic kidney disease of unknown origin (CKDu): report from the International Society of Nephrology Consortium of Collaborators on CKDu. Kidney International, 103 (1), 6-12.
- RECOVERY Collaborative Group. (2022). Aspirin in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. Lancet, 399 (10320), 143-151.
- Cleary F, Kim L, Caplin B, et al. (2022). Association between practice coding of chronic kidney disease (CKD) in primary care and subsequent hospitalisations and death: a cohort analysis using national audit data. BMJ open, 12 (10).
- RECOVERY Collaborative Group. (2022). Baricitinib in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial and updated meta-analysis. The Lancet, 400 (10349), 359-368.
- RECOVERY Collaborative Group. (2022). Casirivimab and imdevimab in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. The Lancet, 399 (10325), 665-676.
- O'Callaghan-Gordo C., Arjona L, Brocal F, Caplin B, et al. (2022). Heat stress and incidence of acute kidney injury among agricultural workers in Spain. The Lancet.
- Ashby DR, Caplin B, Corbett RW, et al. (2022). Outcome and effect of vaccination in SARS-CoV-2 Omicron infection in hemodialysis patients: a cohort study. Nephrology Dialysis Transplantation, gfac209.
- Ashby DR, Caplin B, Corbett RW, et al. (2022). The severity of COVID-19 after Vaccination among Hemodialysis Patients: An Observational Cohort Study. Clin J Am Soc Nephrol.
- RECOVERY Collaborative Group. (2021). Azithromycin in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. The Lancet, 397 (10274).
- Ruiz-Alejos A, Caplin B, Miranda JJ, et al. (2021). CKD and CKDu in northern Peru: a cross-sectional analysis under the DEGREE protocol. BMC Nephrology, 22 (1).
- RECOVERY Collaborative Group. Colchicine in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. (2021) The Lancet Respiratory Medicine.
- RECOVERY Collaborative Group. (2021). Convalescent plasma in patients admitted to hospital with COVID-19 (RECOVERY): a randomised controlled, open-label, platform trial. The Lancet, 397 (10289), 2049-2059.
- Dritsoula A, Kislikova M, Oomatia, Caplin B, et al. (2021). Epigenome-wide methylation profile of chronic kidney disease-derived arterial DNA uncovers novel pathways in disease-associated cardiovascular pathology. Epigenetics, 1-11.
- Hendra H, Caplin B, Salama AD, et al. (2021). Identifying prognostic risk factors for poor outcome following COVID-19 disease among in-centre haemodialysis patients: role of inflammation and frailty. Journal of Nephrology.
- Caplin B, Ashby D, Salama AD, et al. (2021). Risk of COVID-19 Disease, Dialysis Unit Attributes, and Infection Control Strategy among London In-Center Hemodialysis Patients. Clinical Journal of the American Society of Nephrology.
- The RECOVERY Collaborative Group. (2020). Dexamethasone in Hospitalised Patients with Covid-10. The New England Journal of Medicine.
- The RECOVERY Collaborative Group. (2020). Effect of Hydroxychloroquine in Hospitalized Patients with Covid-19. New England Journal of Medicine, 383 (21).
- Cleary F, Prieto-Merino D, Caplin B, et al. (2020). Feasibility of evaluation of the natural history of kidney disease in the general population using electronic healthcare records. Clinical Kidney Journal.
- Mccafferty K, Caplin B, Knight S, et al. (2020). HEROIC: a 5-year observational cohort study aimed at identifying novel factors that drive diabetic kidney disease: rationale and study protocol. BMJ Open, 10 (9), e033923.
- Salama AD, Caplin B. (2020). Lupus Nephritis and Chronic Kidney Disease. J Rheumatol, 47 (9), 1303-1304.
 Close
Close