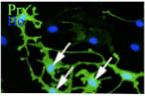
Research Interests
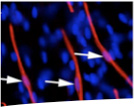
In 1994, our laboratory identified the Schwann cell precursor, a finding that provided the missing developmental link between neural crest cells and Schwann cells (Jessen et al., Neuron 12:509-527, 1994). We were also the first to analyse non-myelinating Schwann cells in molecular terms and to relate their molecular phenotype to the other cells in the lineage. Together with the identification of the Schwann cell precursor this made it possible to establish a general outline of the Schwann cell lineage, a widely used conceptual framework for developmental studies of Schwann cells (Jessen and Mirsky, Trends Neurosci 22: 402-410, 1999; Jessen and Mirsky, Nat Rev Neurosci 6:671-682, 2005; Mirsky et al., J Per Nerv Sys 13: 122-135, 2008).
The group provided the first evidence that maintenance of myelin-specific lipids and proteins by Schwann cells required appropriate axonal signals (Mirsky et al., J Cell Biol 83: 483-494, 1980). We also demonstrated the key importance of neuregulin-1 for the survival of the Schwann cell precursor, identified a switch from juxtacrine to autocrine survival control during embryonic Schwann cell development and determined the molecular identity of autocrine Schwann cell survival factors that enable Schwann cells to survive in the absence of axons (Dong et al., Neuron 15:585-596, 1995; J Neurosci Res 56:334-348, 1999; Meier et al. J Neurosci 19:3847-3859, 1999). We provided the first identification of a growth factor receptor (TGFβRII) involved in the control of Schwann cell proliferation and survival in vivo (D’Antonio et al.,J Neurosci 26:8417-8427,2006), and the first in vivo proof of the idea that Schwann cells provide secreted trophic signalling proteins (Dhh) that are essential for normal nerve structure and function (Parmantier et al. Neuron 23:713-724,1999; Sharghi-Namini et al., Neurosci 26:6364-6376, 2006)
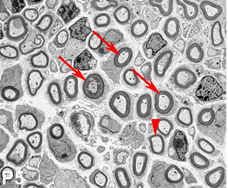 In studies on the key pro-myelin transcription factor Krox-20 we showed that enforced Krox-20 expression induces a broad spectrum of phenotypic changes associated with the transition from immature proliferating cells to quiescent myelinating cells (Parkinson et al., Mol Cell Neurosci 23:13-27, 2003; J Cell Biol 164:385-394, 2004). Thus Krox-20 cell-autonomously inactivates the mitogenic response to the major axonal mitogen neuregulin-1 and protects Schwann cells from death in response to TGF-beta, a molecule that we have shown to act as a Schwann cell killing signal in nerve development (Parkinson et al., J Neurosci 21:8572-8585, 2001; D’Antonio et al.,J Neurosci 26:8417-8427,2006). Not only in Schwann cells but also in 3T3 fibroblasts, which are unrelated to Schwann cells, Krox-20 blocks both proliferation and death and also activates the myelin genes P0 and periaxin. Krox-20 therefore shows properties in common with master regulatory genes in other tissues (Parkinson et al., J Cell Biol 164:385-394, 2004). A major function of Krox-20 is to suppress the c-Jun N-terminal kinase (JNK)/c-Jun pathway and expression of the c-Jun protein. This is significant because we have demonstrated that c-Jun is a negative regulator of myelination and that suppression of c-Jun is required for myelination to proceed (Parkinson et al., J Cell Biol 164:385-394, 2004; J Cell Biol 181: 625-637, 2008).
In studies on the key pro-myelin transcription factor Krox-20 we showed that enforced Krox-20 expression induces a broad spectrum of phenotypic changes associated with the transition from immature proliferating cells to quiescent myelinating cells (Parkinson et al., Mol Cell Neurosci 23:13-27, 2003; J Cell Biol 164:385-394, 2004). Thus Krox-20 cell-autonomously inactivates the mitogenic response to the major axonal mitogen neuregulin-1 and protects Schwann cells from death in response to TGF-beta, a molecule that we have shown to act as a Schwann cell killing signal in nerve development (Parkinson et al., J Neurosci 21:8572-8585, 2001; D’Antonio et al.,J Neurosci 26:8417-8427,2006). Not only in Schwann cells but also in 3T3 fibroblasts, which are unrelated to Schwann cells, Krox-20 blocks both proliferation and death and also activates the myelin genes P0 and periaxin. Krox-20 therefore shows properties in common with master regulatory genes in other tissues (Parkinson et al., J Cell Biol 164:385-394, 2004). A major function of Krox-20 is to suppress the c-Jun N-terminal kinase (JNK)/c-Jun pathway and expression of the c-Jun protein. This is significant because we have demonstrated that c-Jun is a negative regulator of myelination and that suppression of c-Jun is required for myelination to proceed (Parkinson et al., J Cell Biol 164:385-394, 2004; J Cell Biol 181: 625-637, 2008).
Recently, we have established that another transcription regulator, Notch, has multiple and stage-specific regulatory functions in developing and adult Schwann cells that operate right across the lineage, from the Schwann cell precursors in early embryonic nerves to the control of injury responses in mature cells. Notch advances Schwann cell generation, inhibits myelination and drives injury-induced demyelination. Woodhoo et al., 2009, Nature Neuroscience in press).
Current work in the laboratory addresses a group of interlocking issues:
(i) Early Schwann cell development and the biology of the Schwann cell precursor (SCP).
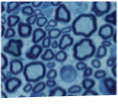
(ii) The molecular regulation of myelination and how this fails in demyelinating disease.
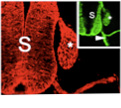
The response of Schwann cells to injury, in particular the mechanisms that drive dedifferentiation and the signals that enable Schwann cells to support nerve regeneration and neuronal survival following injury.
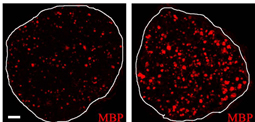 Our studies on c-Jun and Notch have established the existence of negative transcriptional regulation of myelin differentiation (Jessen and Mirsky, Glia 56:1552-1565, 2008), a mechanism that is likely to be particularly important in injured and regenerating nerves. A strong focus of interest is therefore the interplay between Krox-20, c-Jun and Notch. We are studying the capacity of c-Jun and Notch to direct a programme of dedifferentiation, support neuronal survival, and regulate the rate of axon growth. We are also investigating the mechanisms that activate c-Jun and Notch in injured nerves, and in the nerves of a mouse model of the major human demyelinating neuropathy CMT1A. The importance of these control systems in PNS injury is demonstrated by the finding that re-expression of c-Jun in Schwann cells of damaged nerves is essential for axonal regeneration and functional recovery (Jessen KR, Mirsky R, Glia 56:1552-1565, 2008).
Our studies on c-Jun and Notch have established the existence of negative transcriptional regulation of myelin differentiation (Jessen and Mirsky, Glia 56:1552-1565, 2008), a mechanism that is likely to be particularly important in injured and regenerating nerves. A strong focus of interest is therefore the interplay between Krox-20, c-Jun and Notch. We are studying the capacity of c-Jun and Notch to direct a programme of dedifferentiation, support neuronal survival, and regulate the rate of axon growth. We are also investigating the mechanisms that activate c-Jun and Notch in injured nerves, and in the nerves of a mouse model of the major human demyelinating neuropathy CMT1A. The importance of these control systems in PNS injury is demonstrated by the finding that re-expression of c-Jun in Schwann cells of damaged nerves is essential for axonal regeneration and functional recovery (Jessen KR, Mirsky R, Glia 56:1552-1565, 2008).
Collaborators
L Greensmith (UCL); G Raivich (UCL); M Reilly (UCL); S Brandner (UCL); F Baas, Free University Amsterdam, the Netherlands; A Behrens CRUK, London; D Meijer, Erasmus University Medical Center, Rotterdam, The Netherlands; ML Feltri, L Wrabetz, San Raffaele Institute, Milan, Italy; T Honjo, Kyoto University, Japan; D. Parkinson, Peninsula University Medical School, Plymouth; F Radtke, Ludwig Institute for Cancer Research, Lausanne, Switzerland; J. Shen, Harvard University Medical School, Boston, USA; A. Woodhoo, CIC Biogune, Bilbao, Spain.