
|
 |
Neal Skipper (CMMP) - Research
Our research interests mainly concern the properties of liquids,
glasses, interfaces and intercalated materials.
This research involves a variety of experimental
and computational techniques, including neutron and X-ray scattering,
and classical Monte Carlo and molecular dynamics. Examples of some of
our current research projects are given below.
Electronic Solutions
| Electronic Solutions are formed when a metal dissolves in a polar solvent
without chemical reaction, the prototypical solvent being liquid ammonia.
This process reversibly releases excess electrons into the liquid, creating a
solution of fundamental particles! The presence of these electrons results
in liquids that are truly extraordinary, and which have a unique combination of
properties which are yet to be exploited. For example; very low density, very
low viscosity, a deep pseudoeutectic (giving the lowest temperature liquid metals),
a concentration driven metal-nonmetal transition, liquid-liquid phase separation,
highly conducting glassy phases, high electrical conductivity, and exceptionally
high redox reactivity.
|
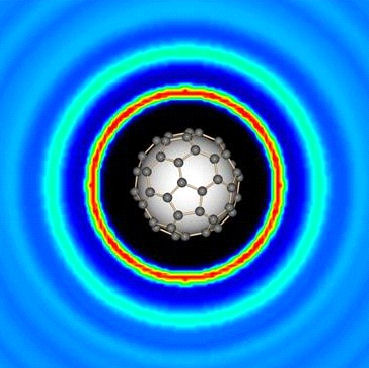
Our recent neutron and X-ray scattering experiments on these liquids have illucidated the structure
and dynamics of these extraordinary liquids, including the solvation of the electron (polaraons and
bipolarons) and the mechanisms of electron delocalisation. In addition, we have used these liquids
as solvents for carbon nanostructures (fullerides) - and shown how C60 is solvated.
Hydrogen Storage
| For fuel cells to successfully reduce the emission of carbon dioxide and other greenhouse gases,
the hydrogen used as fuel needs to be derived from non-polluting renewable sources. We are
currently investigating novel carbon-based materials for reversible hydrogen storage, hopefully up
to and above the 6% by weight required by the automobile industry.
|
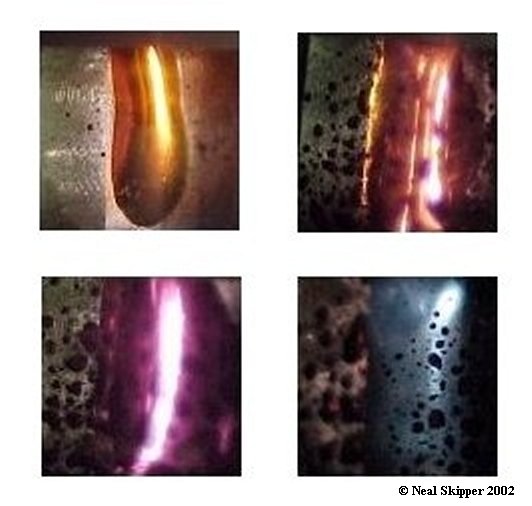
Superconducting Graphite Intercalates
| Low dimensionality is generally considered as a necessary ingredient
for high superconducting transition temperatures. Surprisingly, perhaps,
systems based on graphite have received relatively little attention in this context.
Introducing metal atoms between the carbon layers can
tune the interlayer spacing and charging of the graphite
host through a variety of electronic ground states. One
such ground state is superconductivity3, which is not
present in pure graphite. Here we report the discovery of
superconductivity in the intercalation compounds C6Yb
and C6Ca, with transition temperatures of 6.5 and 11.5 K,
respectively. These critical temperatures are unprecedented
in graphitic systems and have not been explained by a
simple phonon mechanism for the superconductivity. This
discovery has already stimulated several proposals for the
superconducting mechanism that range from coupling by
way of the intercalant phonons through to acoustic plasmons.
It also points towards the potential of superconductivity in
systems such as carbon nanotubes.
|
Confined Organic Molecules
| Understanding and controlling the way in which organic molecules diffuse through
nanometer scale pores is a key problem in environmental science, and in locating
and extracting natural gas and oil from the ground. We are currently using a
combination of neutron scattering and computer modelling to study the diffusion
of simple organic molecules, such as methanol, phenol and glycol, are able to diffuse
through porous media, under sub-surface conditions.
|
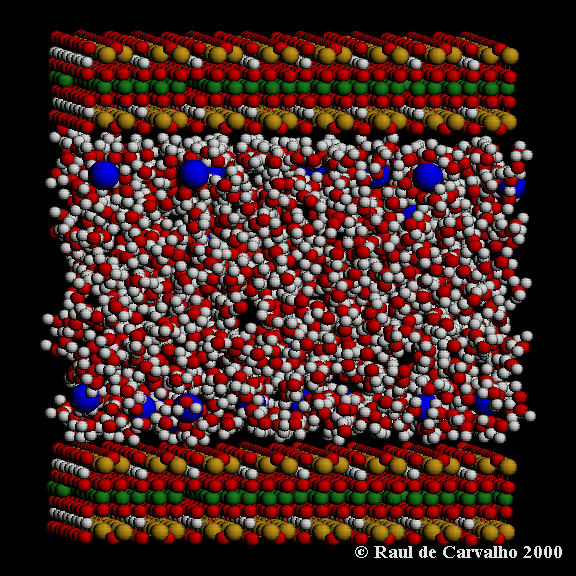
Clay Hydrates
| Swelling 2:1 clays, such as smectite and vermiculite, are widespread in
soils and sedimentary rocks, and have a number of important industrial uses.
They comprise negatively charged mica-like sheets, which are held together by charge
balancing cations, such as Ca2+ and Na+. It is these cations which have a strong tendency
to solvate in the presence of water and polar solvents, thereby forcing the clay sheets
apart. Once expanded, the interlayer pores of clays can accommodate a variety of solute
species, including alcohols, alkanes and organic contaminants. The interlayer pores of
swelling 2:1 clays are therefore an ideal environment in which to study confined fluids,
and are the site of many important hydrological and petrological processes. These
include the diagenetic reactions which are a major source of water in the earth’s crust,
ion exchange in soils and sedimentary rocks, and primary and tertiary migration of
petroleum hydrocarbons. In addition, the hydration and dehydration of expandable clays
is a continuing problem for the construction, waste containment and oil-well drilling
industries. To understand and predict these processes we require a detailed knowledge
of clay-fluid interactions, under sedimentary basin conditions.
|
|
 |





|









