Branching morphogenesis - Dr Marcus Fruttiger's lecture
General points
Even though all organisms are 3 dimensional "objects" metabolic exchange processes occur over 2 dimensional surfaces. In single cell organisms the surface of the organism is sufficient for metabolic exchange with the environment. However, in bigger organisms metabolic exchange surfaces have to be much larger. These exchange surfaces are folded and fragmented so they can be packed inside the body of animals (unlike plants). For example, gas exchange in the human lung occurs across the surface of about 300 million alveoli with a total surface of about 100 m2; nutrient and gas exchange in the human vasculature takes place across the total surface of about 600 m2. Evolution has favored a branched morphology of such metabolically active surfaces, maximizing the active surface and minimizing transport distances. Lungs, kidneys, mammary glands, salivary glands or the vasculature all display a branched morphology.
How to build branched structures
In principle, a branched structure can be built by the iterative use of a few simple subroutines. These are the initiation of a bud, the extension of a bud and the splitting at the end of a bud. With this simple set of morphogenetic units complex structures can be built. The formation of new buds, creating new centers of growth, is important for the overall morphology of the branched structure. For example in the human lung different modes of bud formation occur during different stages of development. In the early phases of bronchi formation new buds arise at the side of an extended bud, a process termed ‘secondary budding’. At later stages new buds form at the end of an extended bud (‘branching’) and in the final stages during alveoli formation terminal sacs are split in several compartments by invagination (‘splitting’).
Drosophila tracheal system
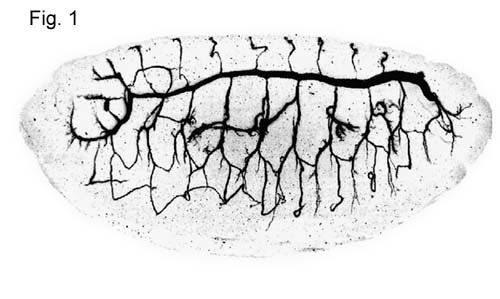 The tracheal system in drosophila
larvae is a relatively simple model system for the study of branched structures
and has provided some amazing insights into the biology of branching
morphology. The system is a tubular network formed by a monolayer epithelium
(Fig.1). Oxygen enters through the spiracular
openings and diffuses freely to the target tissues. The network has three
branching levels: primary branches, secondary branches and blind ending
terminal branches. The first two branching levels display a stereotypical
morphology whereas the terminal branches are not stereotypical. Terminal
branches are very thin cytoplasmic extensions contacting in many tissues almost every single cell. The
reproducible pattern of the primary and secondary branches implies a tight
morphogenetic program responsible for the development of the network.
The tracheal system in drosophila
larvae is a relatively simple model system for the study of branched structures
and has provided some amazing insights into the biology of branching
morphology. The system is a tubular network formed by a monolayer epithelium
(Fig.1). Oxygen enters through the spiracular
openings and diffuses freely to the target tissues. The network has three
branching levels: primary branches, secondary branches and blind ending
terminal branches. The first two branching levels display a stereotypical
morphology whereas the terminal branches are not stereotypical. Terminal
branches are very thin cytoplasmic extensions contacting in many tissues almost every single cell. The
reproducible pattern of the primary and secondary branches implies a tight
morphogenetic program responsible for the development of the network.
Morphogenetic control of the drosophila tracheal system
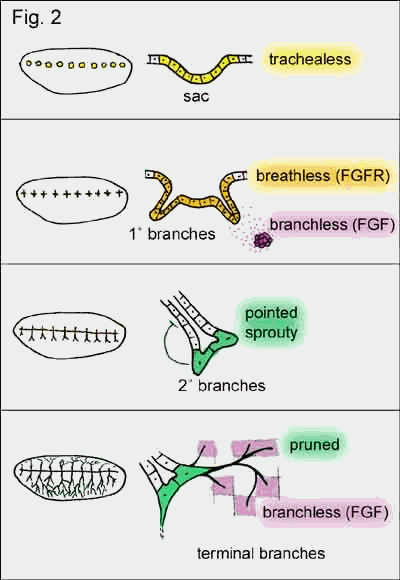 Genetic screens of
drosophila larvae have revealed more than 50 genes involved in the formation of
the tracheal system. Some of the genes are used during each generation of
branch formation whereas others are specifically involved in early or late
stages of the developing network. A gene expressed very early in development is
trachealess, a transcription factor, which appears
in 10 patches bilaterally along the longitudinal axes of the larvae. This
initiates tissue locally to form slight invaginations (sacs) and defines cells
as future components of the tracheal system. Without it no trachea will form
(hence the name). It also induces the expression of breathless in these
sacs, a drosophila ortholog of the mammalian
fibroblast growth factor receptor (FGFR). The ligand
for this receptor is branchless (drosophila ortholog
of FGF) and is dynamically expressed in 6 patches of mesenchymal
tissue around each sac. This diffusible factor induces bud formation and bud
extension from the sacs resulting in 6 primary branches. Some of these branches
fuse forming a longitudinal continues tube (involving a gene called escargot).
The other branches grow closer to the branchless expressing centers
exposing the tip of the growing branch to high concentrations of Branchless.
This high concentration of Branchless induces the expression of pointed
(transcription factor) and sprouty (antagonist
of branchless) at the tip of the branch. Pointed causes the tip
of the branch split (secondary branches) and sprouty
restricts branching to the tip by inhibiting branching further back along the
extended branch. Secondary branches also express pruned (transcription
factor) which is a prerequisite for the formation of terminal branches. These
terminal branches are very thin cellular processes growing towards cells
secreting Branchless. However, this time branchless is not expressed
stereotypical but is induced in any cell experiencing hypoxia. In summary, the
diffusible factor Branchless is used three times in the formation of the
drosophila tracheal system but at each level in a different context. At the
level of primary branches branchless induces bud formation and
extension, at the secondary level high concentrations of Branchless induces the
expression of genes involved in secondary branching and at the level of
terminal branches branchless mediates the need of oxygen-starved cells
for oxygen by promoting and directing the growth of terminal branches.
Genetic screens of
drosophila larvae have revealed more than 50 genes involved in the formation of
the tracheal system. Some of the genes are used during each generation of
branch formation whereas others are specifically involved in early or late
stages of the developing network. A gene expressed very early in development is
trachealess, a transcription factor, which appears
in 10 patches bilaterally along the longitudinal axes of the larvae. This
initiates tissue locally to form slight invaginations (sacs) and defines cells
as future components of the tracheal system. Without it no trachea will form
(hence the name). It also induces the expression of breathless in these
sacs, a drosophila ortholog of the mammalian
fibroblast growth factor receptor (FGFR). The ligand
for this receptor is branchless (drosophila ortholog
of FGF) and is dynamically expressed in 6 patches of mesenchymal
tissue around each sac. This diffusible factor induces bud formation and bud
extension from the sacs resulting in 6 primary branches. Some of these branches
fuse forming a longitudinal continues tube (involving a gene called escargot).
The other branches grow closer to the branchless expressing centers
exposing the tip of the growing branch to high concentrations of Branchless.
This high concentration of Branchless induces the expression of pointed
(transcription factor) and sprouty (antagonist
of branchless) at the tip of the branch. Pointed causes the tip
of the branch split (secondary branches) and sprouty
restricts branching to the tip by inhibiting branching further back along the
extended branch. Secondary branches also express pruned (transcription
factor) which is a prerequisite for the formation of terminal branches. These
terminal branches are very thin cellular processes growing towards cells
secreting Branchless. However, this time branchless is not expressed
stereotypical but is induced in any cell experiencing hypoxia. In summary, the
diffusible factor Branchless is used three times in the formation of the
drosophila tracheal system but at each level in a different context. At the
level of primary branches branchless induces bud formation and
extension, at the secondary level high concentrations of Branchless induces the
expression of genes involved in secondary branching and at the level of
terminal branches branchless mediates the need of oxygen-starved cells
for oxygen by promoting and directing the growth of terminal branches.
Mammalian lung
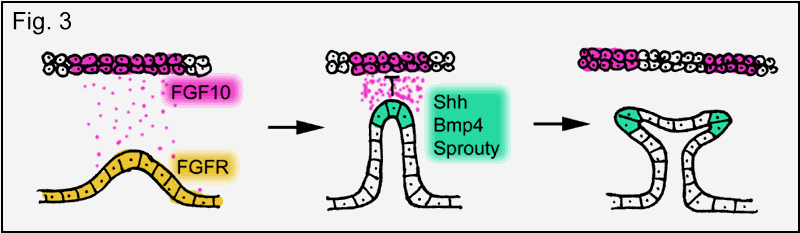 Like most internal
organs the lungs develop from the foregut (in the early embryo a ventral
longitudinal tube). Initially, two buds extend from the foregut resulting in
the left and right bronchus. In the mouse four secondary buds extend from the
two initial branches (three on the right-hand side and one on the left) giving
rise to four lung lobes. The genetic control of these processes is not
completely understood but it has been shown that Gli
genes are involved in the induction of the secondary buds. It is surprising
that also FGF (human ortholog to drosophila
branchless) is crucially involved in mammalian lung formation. Mammals possess
more than one FGF gene. Currently there are more than 20 different FGFs known and many of them have overlapping functions.
However, "knock out mice" lacking FGF10 are
born without lungs and limbs (limbs are also formed from buds!). The function
of FGF in lung development is similar to branchless in drosophila
trachea formation (Fig. 3). The underlying principle is again a mesenchymal-epithelial cell-cell interaction mediated by
FGF. Epithelial cells, expressing FGF receptor, respond to the secretion
of FGF from nearby mesenchyme by bud formation and
bud extension towards the FGF source. Exposure of the branch tip to high
concentrations of FGF induces the expression of secondary genes in the tip such
as bone morphogenetic protein 4 (BMP4), sonic hedgehog (Shh) and a mammalian sprouty ortholog (Sprouty 2),
thus, turning the tips of the bronchial branches into signaling centers. BMP4
inhibits epithelial cell proliferation limiting branch extension. Shh is proposed to inhibit FGF10 expression in the mesenchyme near the tip, which splits FGF10
expression promoting the next round of branching and Sprouty2 (like drosophila sprouty) restricts branching to the tip of
the branch.
Like most internal
organs the lungs develop from the foregut (in the early embryo a ventral
longitudinal tube). Initially, two buds extend from the foregut resulting in
the left and right bronchus. In the mouse four secondary buds extend from the
two initial branches (three on the right-hand side and one on the left) giving
rise to four lung lobes. The genetic control of these processes is not
completely understood but it has been shown that Gli
genes are involved in the induction of the secondary buds. It is surprising
that also FGF (human ortholog to drosophila
branchless) is crucially involved in mammalian lung formation. Mammals possess
more than one FGF gene. Currently there are more than 20 different FGFs known and many of them have overlapping functions.
However, "knock out mice" lacking FGF10 are
born without lungs and limbs (limbs are also formed from buds!). The function
of FGF in lung development is similar to branchless in drosophila
trachea formation (Fig. 3). The underlying principle is again a mesenchymal-epithelial cell-cell interaction mediated by
FGF. Epithelial cells, expressing FGF receptor, respond to the secretion
of FGF from nearby mesenchyme by bud formation and
bud extension towards the FGF source. Exposure of the branch tip to high
concentrations of FGF induces the expression of secondary genes in the tip such
as bone morphogenetic protein 4 (BMP4), sonic hedgehog (Shh) and a mammalian sprouty ortholog (Sprouty 2),
thus, turning the tips of the bronchial branches into signaling centers. BMP4
inhibits epithelial cell proliferation limiting branch extension. Shh is proposed to inhibit FGF10 expression in the mesenchyme near the tip, which splits FGF10
expression promoting the next round of branching and Sprouty2 (like drosophila sprouty) restricts branching to the tip of
the branch.
Summary
In order to form a branching structure an iterative process of bud formation, bud extension and branching is required. It is remarkable that FGF-FGFR interactions appear to be a central unit repetitively used to generate the branching morphology in both the drosophila tracheal system and the mammalian lung.
Further reading:
B.L. Hogan, Morphogenesis (1999) Cell 96, 225-233.
R.J. Metzger and M.A. Krasnow, Genetic control of branching morphogenesis (1999) Science 284, 1635-1639.