Vascular
Development
(lecture summary, Marcus Fruttiger)
Introduction
The vasculature is the earliest organ to develop. Unlike other organs it has to be functional throughout development and therefore has to constantly adapt to the changing requirements of embryogenesis. Anybody who has ever attempted DIY plumbing at home will appreciate the unforgiving nature of a pressurized system. A single leak will affect the entire system with disastrous consequences. Similarly, vascular malformations during development tend to be catastrophic. As soon as an embryo grows bigger than about 2mm it critically depends on a functional vasculature because passive diffusion is not sufficient to supply all cells with oxygen and nutrients. This also holds true for an adult body, which is permeated by capillaries down to every millimeter of tissue (with the exception of cartilage and the cornea). These capillaries are supplied by a hierarchical network of vessels. Larger vessels develop in a stereotypical fashion (they have names!) and are under the control of morphogenetic programs. The patterning of small vessels and capillaries is stochastic and regulated by oxygen supply and demand (similar to the Drosophila tracheal system).
Vessel
morphology
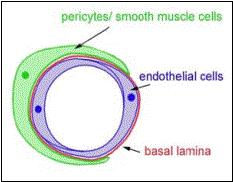 The
two most important vascular cell types are vascular endothelial cells and mural
cells. Vascular endothelial cells form a monolayer throughout the entire
vasculature. They are polarized cells with an apical surface (towards vessel
lumen) and a basal surface (towards “outside”), which is surrounded by a basal
lamina (or basement membrane). Mural cells wrap around this structure and are
contractile cells which regulate vessel diameter and consequently blood flow.
On large vessels they are multi layered and referred to as smooth muscle cells.
On smaller vessels mural cells are more sparse and usually referred to as
pericytes.
The
two most important vascular cell types are vascular endothelial cells and mural
cells. Vascular endothelial cells form a monolayer throughout the entire
vasculature. They are polarized cells with an apical surface (towards vessel
lumen) and a basal surface (towards “outside”), which is surrounded by a basal
lamina (or basement membrane). Mural cells wrap around this structure and are
contractile cells which regulate vessel diameter and consequently blood flow.
On large vessels they are multi layered and referred to as smooth muscle cells.
On smaller vessels mural cells are more sparse and usually referred to as
pericytes.
Formation of blood vessels
Blood vessels and blood arise from common precursor cells (hemangioblasts) which differentiate into blood cell precursors and vascular precursors (angioblasts). These angioblasts migrate, coalesce into cords and form a lumen. This process of vessel formation is called vasculogenesis and is dominant in very early embryogenesis. For example the dorsal aortas and the cardinal veins are formed by vasculogenesis. The sprouting from preexisting vessels is called angiogenesis and the brain is a good example of an organ which is vascularized by this process.
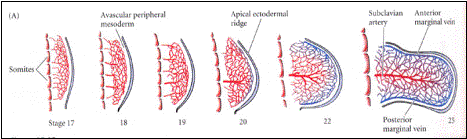 Unlike
other branched organs such as the nervous system or the lungs the vasculature can not develop by a
straightforward tree-like growth, because it is necessary to maintain
functional circulation throughout development. Therefore, larger vessels
develop from simple networks which undergo extensive remodeling. For example
the subclavian artery (main artery going into arm/wing) emerges from a
capillary network which penetrates the limb bud. In summary, there are three
major morphogenetic processes in vascular development: vasculogenesis,
angiogenesis and vascular remodeling.
Unlike
other branched organs such as the nervous system or the lungs the vasculature can not develop by a
straightforward tree-like growth, because it is necessary to maintain
functional circulation throughout development. Therefore, larger vessels
develop from simple networks which undergo extensive remodeling. For example
the subclavian artery (main artery going into arm/wing) emerges from a
capillary network which penetrates the limb bud. In summary, there are three
major morphogenetic processes in vascular development: vasculogenesis,
angiogenesis and vascular remodeling.
Important
genes in vascular development
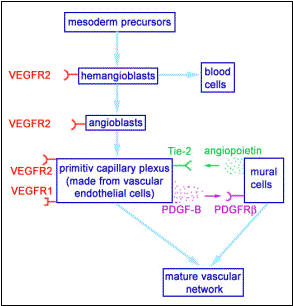 Vascular
endothelial growth factor (VEGF) is the key molecule in vascular development.
VEGF is a survival factor and a mitogen for vascular endothelial cells. Knock
out mice which have the gene for VEGF deleted do not develop any vascular
endothelial cells at all and die at an early embryonic age. There are four
different VEGF genes (VEGF A-D) and four different receptors for VEGF (VEGFR
1-4) which all have different functions (VEGF-C and VEGFR3 are crucial for the
development of the lymph system). For vascular development VEGF-A (generally
referred to just as VEGF), VEGFR1 and VEGFR2 are the most important genes. They
are crucial for the development of the vascular endothelial cell lineage and
for the formation of early capillary net-works. For the maturation and
remodeling of these networks interactions between mural cells and vascular
endothelial cells are required. The cross talk between these two cells types is
in part understood and is mediated by platelet derived growth factor B
(PDGF-B), angiopoietin-1 and their receptors (PDGFRb and Tie-2).
Vascular
endothelial growth factor (VEGF) is the key molecule in vascular development.
VEGF is a survival factor and a mitogen for vascular endothelial cells. Knock
out mice which have the gene for VEGF deleted do not develop any vascular
endothelial cells at all and die at an early embryonic age. There are four
different VEGF genes (VEGF A-D) and four different receptors for VEGF (VEGFR
1-4) which all have different functions (VEGF-C and VEGFR3 are crucial for the
development of the lymph system). For vascular development VEGF-A (generally
referred to just as VEGF), VEGFR1 and VEGFR2 are the most important genes. They
are crucial for the development of the vascular endothelial cell lineage and
for the formation of early capillary net-works. For the maturation and
remodeling of these networks interactions between mural cells and vascular
endothelial cells are required. The cross talk between these two cells types is
in part understood and is mediated by platelet derived growth factor B
(PDGF-B), angiopoietin-1 and their receptors (PDGFRb and Tie-2).
Oxygen
sensing
Similar to branchless in drosophila, VEGF is regulated by oxygen. Most cells secrete VEGF when they experience hypoxia. This will promote the growth of blood vessels towards the hypoxic region. With the arrival of blood vessels (and blood) normoxia is established and VEGF production is down regulated. But how can cells measure the concentration of oxygen?
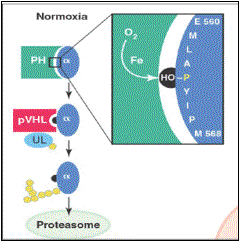 The
key molecule in oxygen sensing is the transcription factor hypoxia induced
factor-1 alpha (HIF-1a) which can
directly activate the expression of VEGF. Under normoxic conditions HIF-1a is constantly produced and constantly
ubiqui-tinated (ubiquitination
is the addition of a tag to proteins which targets them to the proteasome, the
cell’s internal rubbish bin). This ubiquitination is mediated by a protein called Von Hippel-Lindau
protein (pVHL). However, pVHL can only do this when a particular proline
residue in HIF-1a is hydroxylated. This
hydroxylation is carried out by a particular prolyl hydroxylase which is only
active under normoxic conditions. When the oxygen concentration drops this
enzyme is no longer efficient, the proline residue in HIF-1a doesn’t get hydroxylated and pVHL can no
longer mediate ubiquitination of HIF-1a which then accumulates in the cell and
activates VEGF expression.
The
key molecule in oxygen sensing is the transcription factor hypoxia induced
factor-1 alpha (HIF-1a) which can
directly activate the expression of VEGF. Under normoxic conditions HIF-1a is constantly produced and constantly
ubiqui-tinated (ubiquitination
is the addition of a tag to proteins which targets them to the proteasome, the
cell’s internal rubbish bin). This ubiquitination is mediated by a protein called Von Hippel-Lindau
protein (pVHL). However, pVHL can only do this when a particular proline
residue in HIF-1a is hydroxylated. This
hydroxylation is carried out by a particular prolyl hydroxylase which is only
active under normoxic conditions. When the oxygen concentration drops this
enzyme is no longer efficient, the proline residue in HIF-1a doesn’t get hydroxylated and pVHL can no
longer mediate ubiquitination of HIF-1a which then accumulates in the cell and
activates VEGF expression.
Clinical implications
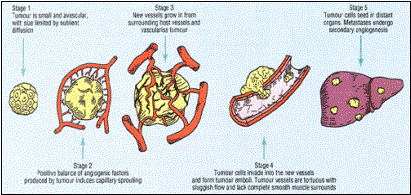 Similar
to embryos, any tumor “wanting” to grow bigger than 2mm needs functional blood
vessels. In order to achieve this, tumor cells have to undergo a so-called
angiogenic switch, mutations which favor blood vessel growth. This will supply
the tumor with oxygen and nutrients but also provides a route for the spreading
of metastasis. If it were possible to prevent tumors from being vascularized
cancer could probably be cured. Judah Folkman has postulated this hypothesis
since the 1970’s. He also discovered the first natural inhibitors of
angiogenesis. These molecules occur naturally and are normally in balance with
promoters of angiogenesis. In 1998 Folkman managed a proof of principle by
demonstrating that injections of angiostatin
(a naturally occurring angiogenesis inhibitor) can suppress the growth of
tumors in mice. Currently, every major drug company has an anti-angiogenesis
program, but whether these activities will translate into a cure for cancer in
humans remains elusive.
Similar
to embryos, any tumor “wanting” to grow bigger than 2mm needs functional blood
vessels. In order to achieve this, tumor cells have to undergo a so-called
angiogenic switch, mutations which favor blood vessel growth. This will supply
the tumor with oxygen and nutrients but also provides a route for the spreading
of metastasis. If it were possible to prevent tumors from being vascularized
cancer could probably be cured. Judah Folkman has postulated this hypothesis
since the 1970’s. He also discovered the first natural inhibitors of
angiogenesis. These molecules occur naturally and are normally in balance with
promoters of angiogenesis. In 1998 Folkman managed a proof of principle by
demonstrating that injections of angiostatin
(a naturally occurring angiogenesis inhibitor) can suppress the growth of
tumors in mice. Currently, every major drug company has an anti-angiogenesis
program, but whether these activities will translate into a cure for cancer in
humans remains elusive.
Further reading
Textbook:
Gilbert
SF: Developmental Biology (sixth edition), Chapter 15
Reviews:
D.
Hanahan 1997 Science; 277:48-50
Zhu
H and Bunn HF 2001 Science; 292:449-51
Yancopoulos
GD et al. 1998 Cell 93:661-4
Risau
W 1997 Nature 386:671-4.
Biography:
Dr
Folkman's War: Angiogenesis and the Struggle to Defeat Cancer. Robert Cooke
