publications and PDFs lab protocols
request-a-mouse UCL
Home
Richardson Lab at UCL Neural development, plasticity and repair
William D
Richardson PhD FMedSci FRS
Wolfson Institute for Biomedical Research
tel +44 (0)20 7679 6729
assistant:
Andrea Goncalves andrea.goncalves@ucl.ac.uk
lab manager: Matthew Grist m.grist@ucl.ac.uk
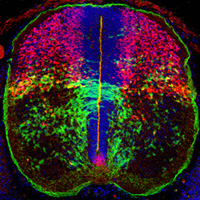
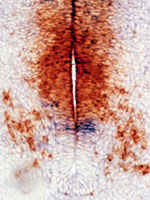
Cell-cell interactions in the developing central
nervous system The vertebrate central nervous
system (CNS) is immensely complicated, yet it has simple
beginnings. The huge number and variety of cells in the
mature CNS all develop from a much smaller number of
precursor (stem) cells in the embryonic neural tube.
Two of the central questions of neurodevelopment - and
development in general - are: 1) How do stem cells select
their future fates? 2) How do stem cells generate their
differentiated progeny in correct numerical proportion to
each other and to the size of the embryo as a whole? We
have addressed these issues, focusing on the development
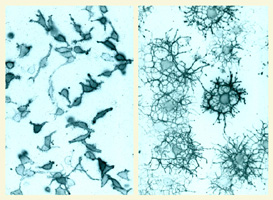
of glial progenitor cells in the CNS. We have taken a
multidisciplinary approach including primary cell culture,
in situ methods and genetic manipulation in mice (e.g. Li
et al., 2011, Tsai et al., 2012)
William D Richardson short CV
Xiao, L., Ohayon, D, McKenzie, I.A., Sinclair-Wilson, A., Wright, J.L., Fudge, A.D., Emery, B., Li, H. and Richardson, W.D. (2016). Rapid production of new oligodendrocytes is required in the earliest stages of motor-skill learning. Nat Neurosci 19, 1210-1217.
*McKenzie, I.A., *Ohayon, D., Li, H., Paes de Faria, J., Emery, B., Tohyama, K. and Richardson, W.D. (2014). Motor skills learning requires active central myelination. Science 346, 318-322. doi:10.1126/science.1254960 * equal contributions
Young, K.M., Psachoulia, K., Tripathi, R.B., Dunn, S.-J., Cossell, L., Attwell, D., Tohyama, K. and Richardson, W.D. (2013). Oligodendrocyte dynamics in the healthy adult CNS: evidence for myelin remodelling. Neuron 77, 873-885.
Tsai, H.-H., Li, H., Fuentealba, L., Molofsky, A.V., Taveira‑Marques, R., Zhuang, H., Tenney, A., Murnen, A.T., Fancy, S.P.J., Merkle, F., Kessaris, N., Alvarez‑Buylla, A.*, Richardson, W.D.* and Rowitch, D.H.* (2012). Regional astrocyte allocation regulates CNS synaptogenesis and repair. Science 337, 358-362. *joint senior authors
Li, H., Paes de Faria, J., Andrew, P. Nitarska, J. and Richardson, W.D. (2011). Phosphorylation regulates OLIG2 cofactor choice and the motor neuron-oligodendrocyte fate switch. Neuron 69, 918-929.
Tripathi, R.B., Rivers, L.E., Jamen, F. Young, K.M. and Richardson, W.D. (2010). NG2 glia generate new oligodendrocytes but few astrocytes in a murine experimental autoimmune encephalomyelitis model of demyelinating disease. J. Neurosci. 30, 16383-16390.
Zawadzka, M., Rivers, L., Fancy, S.P.J., Zhao, C.,
Tripathi, R., Jamen, F., Young, K.M.,Goncharevich, A.,
Pohl, H., Rizzi, M., Rowitch, D.H., Kessaris, N., Suter,
U., *Richardson, W.D. and *Franklin, R.J.M. (2010).
CNS-resident glial progenitor/stem cells produce Schwann
cells as well as oligodendrocytes during repair of CNS
demyelination. Cell Stem Cell 6, 578-590. *
joint senior authors
Rivers, L.E., Young, K.M., Rizzi, M.,
Jamen, F., Psachoulia, K., Wade, A., Kessaris, N. and
Richardson, W.D. (2008). PDGFRA/ NG2-positive
glia generate myelinating oligodendrocytes and
piriform projection neurons in adult mice.
Nature Neuroscience 11, 1392-1401.