Ammonia toxicity
Ammonia is highly toxic. Normally blood ammonium concentration is < 50 µmol
/L, and an increase to only 100 µmol /L can lead to disturbance of consciousness.
A blood ammonium concentration of 200 µmol /L is associated with coma
and convulsions.
200 µmol /L is far too low a concentration
of ammonium to affect plasma pH or the normal transport of sodium and potassium
ions across nerve cell membranes.
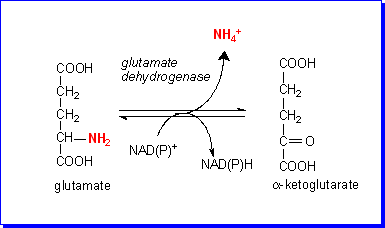 The explanation
of the toxicity of such (relatively) low concentrations of ammonium lies with
the enzyme glutamate dehydrogenase. This enzyme catalyses the oxidative deamination
of glutamate to ammonium and ketoglutarate; the reaction is readily reversible,
and the direction of reaction (towards deamination of glutamate or glutamate
formation) depends on the relative concentrations of the various substrates.
As the concentration of ammonium rises, so the reaction proceeds in the direction
of formation of glutamate from ketoglutarate.
The explanation
of the toxicity of such (relatively) low concentrations of ammonium lies with
the enzyme glutamate dehydrogenase. This enzyme catalyses the oxidative deamination
of glutamate to ammonium and ketoglutarate; the reaction is readily reversible,
and the direction of reaction (towards deamination of glutamate or glutamate
formation) depends on the relative concentrations of the various substrates.
As the concentration of ammonium rises, so the reaction proceeds in the direction
of formation of glutamate from ketoglutarate.
The effect of forming glutamate from ketoglutarate is to deplete the mitochondrial
pool of ketoglutarate, which is a key intermediate in the citric acid cycle.
As a result, the rate of citric acid cycle activity falls, so reducing very
considerably the rate of formation of ATP.
It is this lack of ATP that affects ion transport across nerve cell membranes,
so resulting in disturbance, then loss, of consciousness.
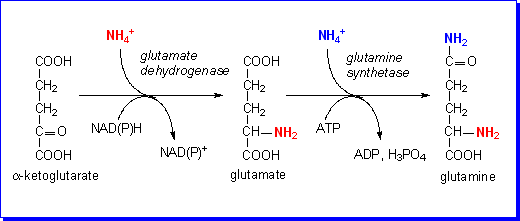
Formation of glutamine
Ammonium is produced in most cells of the body, as a result of deamination
of amino acids and amines. It is exported into the bloodstream as glutamine,
formed by the actions of glutamate dehydrogenase, to form glutamate from ketoglutarate
and ammonium, then glutamine synthetase, forming glutamine from glutamate and
ammonium.
Treatment of ammonia intoxication
Ammonia intoxication occurs when blood ammonium rises because the capacity
to detoxify it by formation of glutamate and glutamine has been exceeded. Sometimes,
in infants whose blood ammonium has risen dangerously high (commonly as a result
of a genetic disease affecting amino acid metabolism), the only emergency treatment
is a more-or-less complete exchange blood transfusion.
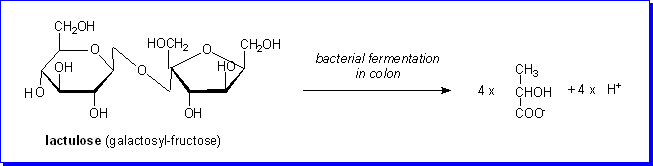
In less critically urgent cases of ammonia intoxication (sometimes in infants,
sometimes in adults with liver failure) the treatment of choice is administration
of the disaccharide lactulose, either orally or by rectal infusion. Lactulose
is a synthetic disaccharide that is not hydrolysed by intestinal enzymes, but
(if given orally) passes into the large intestine, where it is a substrate for
bacterial fermentation to lactate.
.
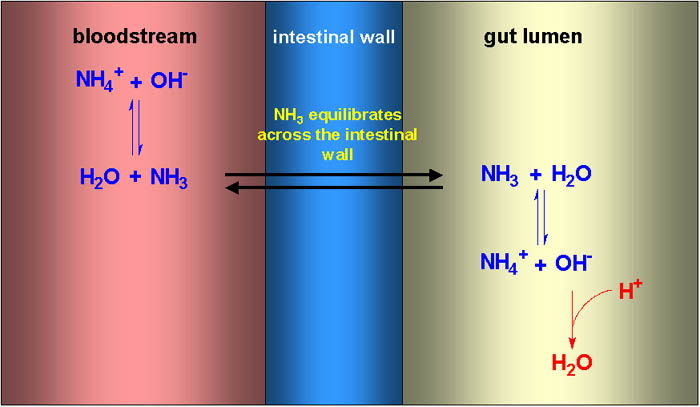
Ammonia will cross the intestinal wall, since it is lipid soluble, but ammonium
ions cannot. At physiological pH the equilibrium between ammonia and ammonium
in the bloodstream is well towards ammonium ions, but there is always a small
amount of ammonia. This can diffuse across the intestinal wall into the lumen
of the gut. Here the result of fermentation of lactulose to lactate is a significant
fall in pH, resulting in a shift of the equilibrium further towards ammonium
ions, which cannot cross the intestinal wall. As a result, ammonium ions are
trapped in the intestinal lumen, and there is a nett flux of ammonia form the
bloodstream into the intestinal lumen.
 The explanation
of the toxicity of such (relatively) low concentrations of ammonium lies with
the enzyme glutamate dehydrogenase. This enzyme catalyses the oxidative deamination
of glutamate to ammonium and ketoglutarate; the reaction is readily reversible,
and the direction of reaction (towards deamination of glutamate or glutamate
formation) depends on the relative concentrations of the various substrates.
As the concentration of ammonium rises, so the reaction proceeds in the direction
of formation of glutamate from ketoglutarate.
The explanation
of the toxicity of such (relatively) low concentrations of ammonium lies with
the enzyme glutamate dehydrogenase. This enzyme catalyses the oxidative deamination
of glutamate to ammonium and ketoglutarate; the reaction is readily reversible,
and the direction of reaction (towards deamination of glutamate or glutamate
formation) depends on the relative concentrations of the various substrates.
As the concentration of ammonium rises, so the reaction proceeds in the direction
of formation of glutamate from ketoglutarate.

