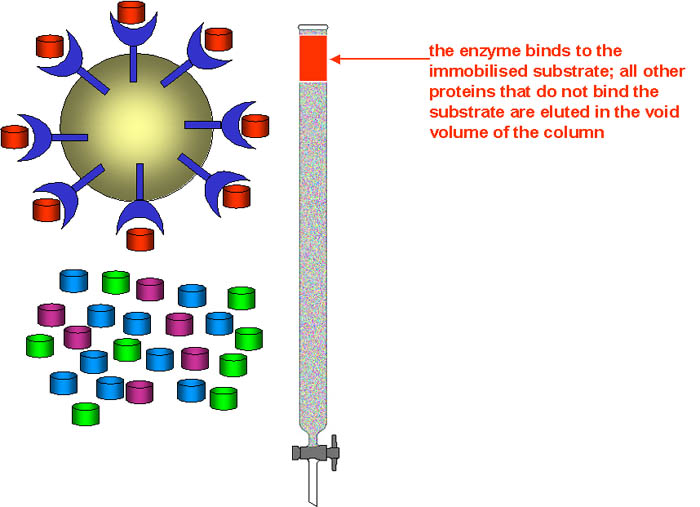
The principle of affinity chromatography is that the stationary phase consists of a support medium (e.g. cellulose beads) on which the substrate (or sometimes a coenzyme) has been bound covalently, in such a way that the reactive groups that are essential for enzyme binding are exposed. As the mixture of proteins is passed through the chromatography column, those proteins that have a binding site for the immobilised substrate will bind to the stationary phase, while all otter proteins will be eluted in the void volume of the column.
Once the other proteins have all been eluted, the bound enzyme(s) can be eluted in various ways:

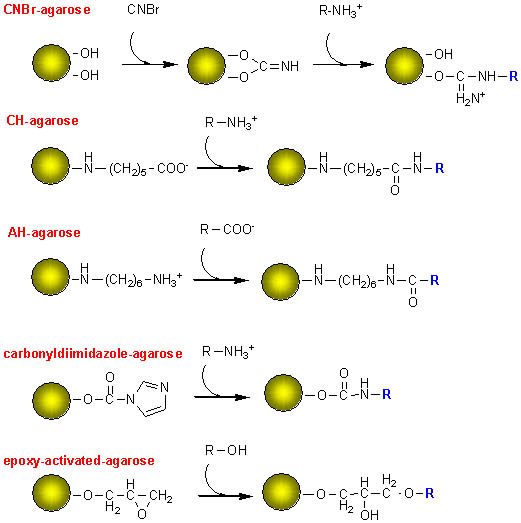 There
are several different activated agarose gels that can be used to attach ligands;
CNBr-agarose is easy to use for the attachment of amines, but does not have
a long spacer between the gel beads and the bound ligand, so that protein binding
may be sterically hindered.
There
are several different activated agarose gels that can be used to attach ligands;
CNBr-agarose is easy to use for the attachment of amines, but does not have
a long spacer between the gel beads and the bound ligand, so that protein binding
may be sterically hindered.
Aminohexanoic acid-agarose (CH-agarose, for reaction with amines) and diaminohexane-agarose (AH-agarose, for reaction with carboxylic acids)) have relatively long methylene chains that keep the ligand a significant distance from the gel beads.
An alternative reagent for attaching amines is carbonyldiimadazole-agarose, and epoxy-activated agarose is used for alcohols.