
The effect of temperature on the rate of an enzyme-catalysed reaction is the result of two opposing factors:

In the graph above the enzyme was incubated at various temperatures for 10 minutes,
and the amount of product formed was plotted against temperature. The enzyme
showed maximum activity at about 55 °C. In the graph below the same enzyme
was incubated at various temperatures for just 1 minute and the amount of product
formed was again plotted against temperature. Now the increased activity with
increasing temperature is more important than the loss of activity due to denaturation
and the enzyme shows maximum activity at 80 °C.
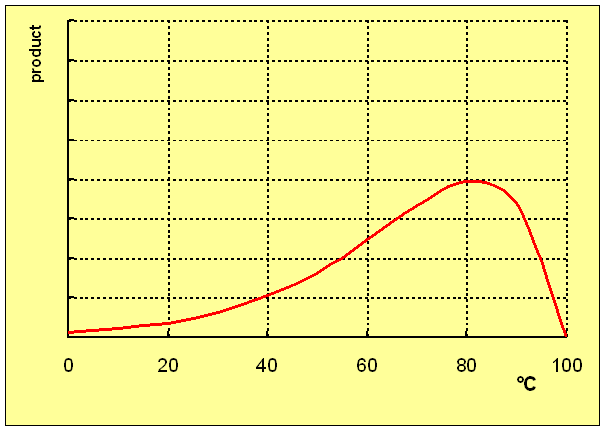
The graph below shows the results of incubating the same enzyme at various temperatures for different times ranging from 1 minute to 10 minutes - the longer the incubation time the lower the temperature at which there is maximum formation of product, because of the greater effect of denaturation of the enzyme.

This means that it is not useful to attempt to determine an 'optimum' temperature for an enzyme-catalysed reaction.
By convention, enzyme activity is determined at 30°C; this is a compromise between mammalian and clinical biochemists, who would expect to work at 37°C, and microbial biochemists, most of whom would expect to work at 20°C.
Investigation of the temperature
dependence of an enzyme can be useful, for example in biotechnology and biochemical
engineering, where there may be operational reasons for working at a relatively
high temperature, so that enzymes with a higher thermal stability are advantageous.
The effect of increasing temperature on the rate of formation of product up to the point at which denaturation begins to reduce the activity of the enzyme can be investigated to determine the activation energy of the reaction. The equation relating the rate of a chemical reaction to temperature was derived empirically by Arrhenius in 1889:
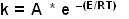
where:
k = the rate constant for the reaction
A = a constant for the reaction
E = the activation energy
R = gas constant = 1.987 cal / deg / mol = 8.31434 joules / deg / mol
T = temperature (°K, = °C + 273)
Taking
logs gives:
log = log A - E / 2.303 * R * T
For temperatures below that at which
there is significant denaturation of the enzyme, if the enzyme is saturated
with substrate, the rate constant is = Vmax, and a
graph of log Vmax against 1 / T will be a straight line with gradient
= - E / 2.303 * R
Activation energy
E = -4.576 * gradient (cal
/ deg / mol)
E = -19.148 * gradient (joules / deg / mol)