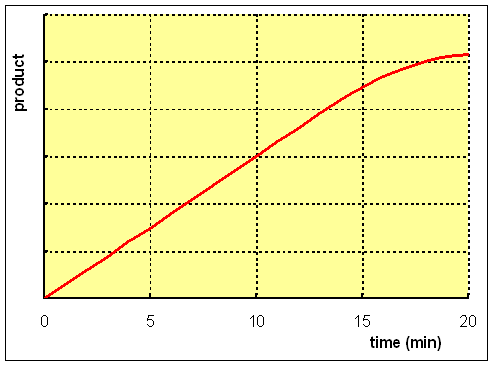
The longer an enzyme is incubated with its substrate, the greater the amount of product that will be formed. However, the rate of formation of product is not a simple linear function of the time of incubation.
All proteins suffer denaturation, and hence loss of catalytic activity, with time. Some enzymes, especially in partially purified preparations, may be noticeably unstable, losing a significant amount of activity over the period of incubation.
If the activity of the enzyme is such that much of the substrate is used up during the incubation, then, even if the concentration of substrate added was great enough to ensure saturation of the enzyme at the beginning of the experiment, it will become inadequate as the incubation proceeds, and the formation of product will decrease.
Enzyme catalysed reactions are reversible. Initially, there is little or no product present, and therefore the reaction proceeds only in the forward direction. However, as the reaction continues, so there is a significant accumulation of product, and there is a significant rate of back reaction. As a result, the rate of formation of product slows down as the incubation proceeds, and if the incubation time is too long, then the measured activity of the enzyme is falsely low.
In some studies, especially when investigating the substrate dependence of the rate of reaction, it is usual to make measurements of the formation of product at relatively short time intervals (say every 10 seconds) for the first minute or so, then plot a graph of the amount of product formed against time, and determine the initial rate of reaction by drawing the tangent to the steepest part of the rate curve. However, in short incubations there can be a considerable error of timing; 1 second is a significant error in a short incubation, but negligible when the incubation time is several minutes. Similarly, in short incubations only a small amount of product has been formed, and analytical errors are magnified when the amount of product is extremely small.
Selecting an appropriate incubation
time depends on a compromise between these various factors.As a general rule,
the incubation should be long enough to permit a moderate amount of product
to be formed, and long enough that the error in timing is insignificant, but
not so long that there is detectable levelling off of the curve. You
need to be sure that when you determine the rate of reaction (in mol of product
formed / minute) the enzyme has been active at a more or less constant rate
throughout your incubation.
