Preprint of paper to be published in Neuropharmacology
Institute of Ophthalmology, University College London, 11-43 Bath Street,
LONDON EC1V 9EL, United Kingdom
and
Lilly Research Centre, Erl Wood Manor
Windlesham, Surrey GU20 6PH
Previous work from this laboratory using a range of phenylglycine antagonists which are active at
Group I mGlu receptors has indicated that such receptors mediate postsynaptic responses of
ventrobasal thalamus neurones to the mGlu agonist (1S,3R)-1-aminocyclopentane-1,3-dicarboxylate (ACPD), and that these receptors are involved in nociceptive responses of thalamic
neurones (Eaton et al. 1993; Salt and Eaton 1994). However, although there is some suggestion
of mGlu1 involvement (Salt and Turner 1998), these data do not allow conclusive determination
of the degree of mGlu1 and mGlu5 mediation of responses. Indeed, although there is a large
degree of mGlu1 mRNA expression in the thalamus (Masu et al. 1991; Shigemoto, Nakanishi,
and Mizuno1992), there is also some expression for mGlu5 mRNA (Abe et al. 1992; Romano,
Van den Pol, and O'Malley 1996), and immunohistochemical studies have revealed receptor
protein for both of these receptor types in the thalamus (Martin et al. 1992; Romano, Van den
Pol, and O'Malley 1996; Romano et al. 1995). The aim of the present study was to investigate a
number of novel mGlu antagonists on the responses of single thalamic neurones to the mGlu
agonists ACPD, (S)-3,5-dihydroxyphenylglycine (DHPG) and CHPG in an attempt to provide
pharmacological evidence for the existence of functional mGlu5 and mGlu1 receptors in the
thalamus, and to evaluate antagonists as a prelude to their use in studies of sensory responses.
These antagonists are the mGlu1 receptor-selective AIDA (Moroni et al. 1997), LY367385
(Clark et al. 1997), the mGlu1/5 receptor-selective LY367366 (Bruno et al. 1999), and the
broader spectrum LY393053 (Baker et al. 1998) (Figure 1).
Thalamic neurones were identified on the basis of their stereotaxic location and responses to
somatosensory stimuli, as described previously (Salt 1987; Salt and Eaton 1994). Regular
repeated cycles (3-5 minute duration) containing two or (in most cases) three agonist ejections
(10-20s durations, 40-95s intervals) were set up and initiated by a computer system. Agonist
ejection parameters were adjusted so as to produce sub-maximal responses. Extracellular action
potentials were gated and timed using the computer system, which could produce peristimulus-histograms of single-neurone activity both online and offline. The effects of antagonists were
assessed by continuous iontophoretic application of antagonists during several cycles of agonist
ejection. Antagonist ejection currents and durations were adjusted so as to achieve selective
antagonism of either ACPD or DHPG responses compared to responses to other agonists
wherever possible, and the rows in the results tables (Tables 1 and 2) represent data obtained
from such comparisons. Responses to agonists were quantified as the number of action potentials
evoked by agonist ejection. The effects of antagonists on these responses were assessed by
calculating the agonist response during antagonist application as a percentage of agonist response
under control conditions. Baseline activity of neurones was not taken into account in these
calculations as this was typically a low contribution of the overall count of action potentials.
The antagonist AIDA was tested on 11 neurones with iontophoretic currents of 40-120nA. This
compound reduced responses to ACPD in all cases, but was also found to reduce responses to
AMPA and /or NMDA to a significant extent (Figure 2). These data are summarised in Table 1.
In view of these data, AIDA was not investigated with other mGlu agonists. In contrast,
LY367385 (5-40nA), LY367366 (5-60nA) and LY393053 (2-20nA) were all able to reduce
responses to ACPD or DHPG with little effect on responses to either NMDA or AMPA (Figure
3), as summarised in Table 1. However, it is noteworthy that LY367385 was able to produce a
slight, but significant, enhancement of responses to NMDA. (Tables 1 and 2). Recovery from the
effects of LY367385 and LY367366 were typically seen within 1-15 minutes after termination of
the iontophoretic ejection, whereas recovery from the effects of LY393053 had a more protracted
time course (up to 25 minutes).
When tested in experiments (11 neurones) where CHPG was included, LY367385 was found to
reduce selectively either ACPD or DHPG compared to CHPG (Figure 4), as summarised in Table
2. In contrast, LY367366 was found to reduce responses to both ACPD and CHPG (Figure 5)
on the neurones tested (Table 2).
It is intriguing that both ACPD and DHPG could be antagonised to a large extent by LY367385,
which is known to be selective for mGlu1 receptors (Clark et al. 1997). As both of these agonists
are known to act at both mGlu1 and mGlu5 receptors, this finding suggests that most of the
excitatory response seen upon iontophoretic application of these mixed agonists is mediated via
mGlu1 receptors. This could be due to the preponderance of mGlu1 receptors compared to
mGlu5 receptors in this brain area (see above). AIDA has also been described as an mGlu1-receptor-selective antagonist (Moroni et al. 1997). However, although this compound did reduce
neuronal responses to ACPD, which is consistent with it being an mGlu receptor antagonist, it
also reduced NMDA and AMPA responses to a significant extent. An effect of AIDA on NMDA
receptors expressed in oocytes has been described recently (Contractor et al. 1998). Furthermore,
an effect of AIDA on NMDA responses has also been found in an in vitro spinal cord study
(Krieger, Grillner, and ElManira 1998), and another study found complex actions of this
compound in the spinal cord (Pinkney et al. 1998). This would appear to limit its usefulness as a
tool in physiological studies, and its use should be approached with caution unless suitable control
experiments are carried out. In contrast, LY367366 and LY393053 appear to be more useful in
that they antagonised postsynaptic responses to the mGlu agonists used in this study whilst having
little effect on responses to NMDA or AMPA. This is consistent with previous findings with
these compounds (Thomas et al. 1998; Baker et al. 1998; Bruno et al. 1999).
In conclusion, the data presented here indicate that in the ventrobasal thalamus there are
functional mGlu1 and mGlu5 receptors which mediate excitatory neuronal responses, and that
these mGlu5 receptors can be selectively activated by CHPG. Furthermore, LY367385 appears
to be useful to antagonise mGlu1 receptors selectively in the thalamus, whereas LY367366 and
LY393053 are more useful as broader-spectrum mGlu receptor antagonists.
Baker SR, Clark BP, Harris JR, Griffey KI, Kingston AE, and Tizzano JP. (1998). LY393675, an a-substituted-cyclobutylglycine, is a potent Group I metabotropic glutamate receptor antagonist. Society for Neuroscience Abstracts 28: 229.16.
Bruno V, Battaglia G, Kingston AE, O'Neill MJ, Catania MV, Di Grezia R, and Nicoletti F. (1999). Neuroprotective activity of the potent and selective mGlu1a metabotropic glutamate receptor antagonist (+)-2-methyl-4-carboxyphenylglycine (LY367385): Comparison with LY367366, an antagonist of mGlu1a and mGlu5 receptors. Neuropharmacology 38: 119-207.
Clark BP, Baker SR, Goldsworthy J, Harris JR, and Kingston AE. (1997). (+)-2-Methyl-4-carboxyphenylglycine (LY367385) selectively antagonises metabotropic glutamate mGluR1 receptors. Bioorganic & Medicinal Chemistry Letters 7: 2777-2870.
Conn PJ, and Pin JP. (1997). Pharmacology and functions of metabotropic glutamate receptors. Annual Review of Pharmacology and Toxicology 37: 207-237.
Contractor A, Gereau, RW, Green T, and Heinemann SF. (1998). Direct effects of metabotropic glutamate receptor compounds on native and recombinant N-methyl-D-aspartate receptors. Proceeding of the National Academy of Sciences (USA) 95: 8969-8974.
Doherty AJ, Palmer MJ, Henley JM, Collingridge GL, and Jane DE. (1997). (R,S)-2-chloro-5-hydroxyphenylglycine (CHPG) activates mGlu5 but not mGlu1 receptors expressed in CHO cells and potentiates NMDA responses in the hippocampus. Neuropharmacology 36: 265-267.
Krieger P, Grillner S, and ElManira A. (1998). Endogenous activation of metabotropic glutamate receptors contributes to burst frequency regulation in the lamprey locomotor network. European Journal of Neuroscience 10: 3333-3342.
Martin LJ, Blackstone CD, Huganir RL, and Price DL. (1992). Cellular localization of a metabotropic glutamate receptor in rat brain. Neuron 9: 259-270.
Masu M, Tanabe Y, Tsuchida K, Shigemoto R, and Nakanishi S. (1991). Sequence and expression of a metabotropic glutamate receptor. Nature 349: 760-765.
Moroni F, Lombardi G, Thomsen C, Leonardi P, Attucci S, Peruginelli F, Albani Torregrossa S, Pellegrini-Giampietro DE, Luneia R, and Pellicciari R. (1997). Pharmacological Characterization of 1-Aminoindan-1,5-dicarboxylic Acid, a Potent mGluR1 Antagonist. Journal of Pharmacology and Experimental Therapeutics 281: 721-729.
Nakanishi S. (1992). Molecular diversity of glutamate receptors and implications for brain function. Science 258: 597-603.
Pinkney JM, Kingston WP, Thomas NK, Jane DE, and Pook PC-K. (1998). Electrophysiological characterisation of (RS)-1-aminoindan-1,5-dicarboxylic acid on the neonatal rat spinal cord preparation. British Journal of Pharmacology 123: 17P.
Romano C, Sesma MA, McDonald CT, O'Malley K, Van den Pol AN, and Olney JW. (1995). Distribution of metabotropic glutamate receptor mGluR5 immunoreactivity in rat brain. Journal of Comparative Neurology 355: 455-469.
Romano C, Van den Pol AN, and O'Malley KL. (1996). Enhanced early developmental expression of the metabotropic glutamate receptor mGluR5 in rat brain: Protein, mRNA splice variants, and regional distribution. Journal of Comparative Neurology 367: 403-412.
Salt TE. (1987). Excitatory amino acid receptors and synaptic transmission in the rat ventrobasal thalamus. Journal of Physiology 391: 499-510.
Salt TE, and Eaton SA. (1991). Excitatory actions of the metabotropic excitatory amino acid receptor agonist, trans-(+)-1-amino-cyclopentane-1,3-dicarboxylate (t-ACPD), on rat thalamic neurones in vivo. European Journal of Neuroscience 3: 1104-1111.
Shigemoto R, Nakanishi S, and Mizuno N. (1992). Distribution of the mRNA for a metabotropic glutamate receptor (mGluR1) in the central nervous system: An in situ hybridization study in adult and developing rat. Journal of Comparative Neurology 322: 121-135.
Thomas NK, Woolley ML, Miller JC, Clark BP, Harris JR, Jane DE, and Watkins JC. (1998). Novel phenylglycines as metabotropic glutamate receptor antagonists. Society for Neuroscience Abstracts 28: 229.15.
|
Antagonist |
ACPD
(% control) |
DHPG
(% control) |
NMDA
(% control) |
AMPA
(% control) |
Current
(nA) |
| A AIDA | 20±3.0*** | - | 52±11.7* | 55±14.5 * | 87±6.9 |
| n=11 | n=11 | n=7 | n=11 | ||
| B | 13±1.9*** | - | 128±8.3*** | 116±10.5 | 22±2.2 |
| n=27 | n=27 | n=18 | n=27 | ||
| C LY367385 | - | 13±3.5* | 124±9.6 | 122±12.8 | 17±1.9 |
| n=6 | n=6 | n=4 | n=6 | ||
| D LY367366 | 20±4.4*** | - | 106±7.7 | 108±7.4 | 27±5.9 |
| n=13 | n=13 | n=7 | n=13 | ||
| E LY393053 | 10±3.0** | - | 96±12.4 | 94±17.2 | 10±2.3 |
| n=10 | n=10 | n=9 | n=10 |
Values are means (± standard error) of agonist responses in the presence of antagonist expressed as percentages of control values, and the mean (± standard error) iontophoretic current of antagonist used to block agonist responses. n values represent numbers of neurones in each group. A - effects of AIDA when tested against ACPD, compared to AMPA and/or NMDA; B- effects of LY367385 when tested against ACPD, compared to AMPA and/or NMDA; C - effects of LY367385 when tested against DHPG, compared to AMPA and/or NMDA; D,E - effects of LY367366 and LY393053, respectively in similar experiments. Values are significantly different from controls (Wilcoxon Signed Rank Test) as indicated: * P < 0.05; ** P < 0.01; *** P < 0.005.
|
Antagonist |
CHPG
(% control) |
DHPG
(% control) |
ACPD
(% control) |
NMDA
(% control) |
Current
(nA) |
| A LY367385 | 78±9.1* | - | 11±2.4** | 128±10.3* | 11±1.8 |
| n=9 | n=9 | n=9 | n=9 | ||
| B LY367385 | 74±6.7 | 25±0.7 | - | 111±10.6 | 10 |
| n=2 | n=2 | n=2 | n=2 | ||
| C LY367366 | 38±3.0* | - | 31±6.7* | 89±7.0 | 15±4.1 |
| n=6 | n=6 | n=6 | n=6 |
Similar data to that in Table 1, but from the subset of neurones where the effects of antagonists on CHPG were directly compared with the effects on either ACPD or DHPG.

Structures of antagonists used in this study.

Peristimulus time histograms (PSTHs) of action potentials (spikes) counted into successive 1000ms epochs showing excitatory responses to iontophoretic ejections of ACPD, AMPA and NMDA at times indicated by the marker bars. The upper record is a control, below which is shown the same set of agonist applications during the co-application of AIDA. The lower record shows recovery from AIDA 15 minutes after the end of the iontophoretic ejection. Note that AIDA reduced responses to all three agonists.
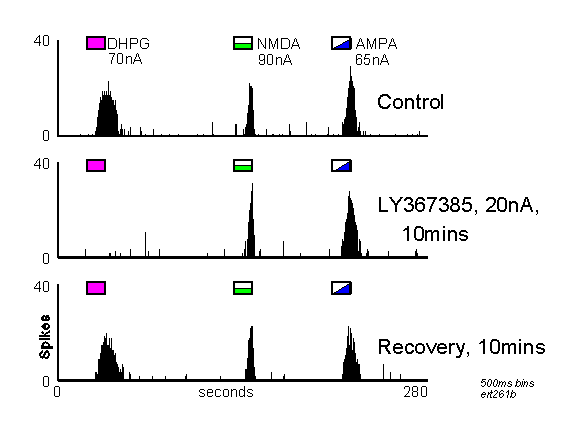
PSTHs showing neuronal responses to DHPG, NMDA and AMPA before, during and after iontophoretic application of LY367385 (For details see Figure 2). Note that this antagonist produced a selective reduction of the responses to DHPG.
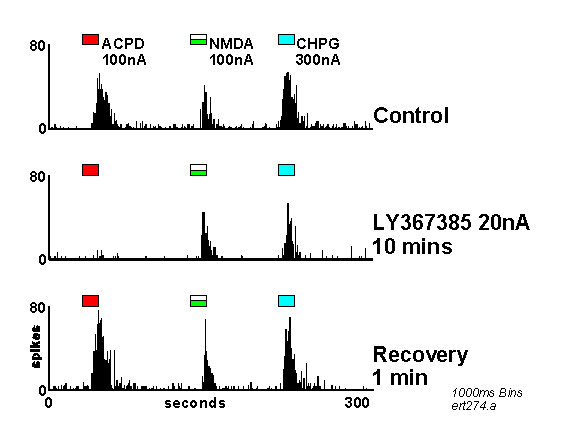
PSTHs showing neuronal responses to ACPD, NMDA and CHPG before, during and after iontophoretic application of LY367385 (For details see Figure 2). Note the selective reduction of the response to ACPD compared to CHPG.

PSTHs showing neuronal responses to CHPG, ACPD and NMDA before, during and after iontophoretic application of LY367366 (For details see Figure 2). Note the reduction of both CHPG and ACPD responses.