
The nitric oxide (NO) system has been the subject of much study since the discovery that it plays an important part in neural signalling (Garthwaite, 1993). NO is a free radical gas which can diffuse across membranes rapidly, thus acting on neural elements which are at some distance from the site of production. One of the modes of action of NO is to stimulate soluble guanylate cyclase, leading to an increase in intracellular cyclic GMP in target cells, and this can then lead to further effects, depending on the cell. NO is synthesised from L-arginine by the action of NO synthase (NOS), with the production of L-citrulline. L-Citrulline is then recycled to L-arginine by argininosuccinate synthetase and argininosuccinase. There is a widespread distribution for NOS within the brain, including in the thalamus and the cerebral cortex, predominantly in neurones but also in astrocytes. Some of these neurones are also immunoreactive for citrulline. Studies have also been carried out on the cellular localisation of the NO precursor L-arginine. Immunohistochemical studies indicate that it is located mainly in glial cells in the CNS, but it is also possible that it is located in neuronal elements. A diversity of cellular compartments suggests not only that NO may be an active end-product but that, as intermediates may move between compartments, L-arginine and L-citrulline might have a signalling function in their own right. This is speculative, but is supported by the release of arginine upon stimulation of pathways in cerebellar slices and in the thalamus in vivo (Do etal., 1994). More recently, the possibility has also arisen that endogenous nitroso-thiols may also participate in these signalling processes by packaging NO in the form of nitrosothiols to facilitate its transfer, prolong its life, and target its delivery (Do et al 1996).
The neuroanatomy and neurophysiology of the thalamic relay nuclei and the related cerebral cortical areas have been thoroughly studied over many decades (Jones 1985). The role of excitatory amino acids and the inhibitory amino acid GABA are now well established in the thalamus.
There is evidence to suggest that NOS in the thalamus is located in the terminals of cholinergic brainstem afferents, although more information is required on this subject and on the localisation of NO and its intermediates. The only anatomical evidence regarding arginine in the thalamus to date indicates that it is localised in glia. Given that arginine is released in vivo upon sensory afferent stimulation, and that L-arginine can enhance sensory synaptic responses via what appears to be an NO-mediated mechanism we have suggested, as a working hypothesis, that NO can be released from the cholinergic terminals and serve a local modulatory role which would be in addition to the release of acetylcholine from the same nerve terminals (evoked by brainstem stimulation) (Do etal., 1994). The release of arginine could represent the activity of NOS and the transfer of arginine from glia to terminals may be required to supply its substrate. The stimulus for the release of arginine is still unclear, but it is likely that sensory afferent activity causes this, either directly or indirectly, possibly via postsynaptic activity in the VB neurones themselves. The latter is supported by our recent finding that Arginine release can be evoked by exogenous NMDA (Salt et al, 1995). NO release in VB may be a local modulatory system which enables rapid enhancement of transmission upon stimulation of sensory afferents: a positive feedback system. If this is the case, then the local NO system could facilitate transmission through a restricted part of the thalamus in order to enhance transmission of an afferent signal which may be deemed important because of its persistence. This hypothesis is strengthened by our recent findings that a number of NO donors can enhance sensory responses of thalamic neurones (Shaw & Salt 1997). Furthermore, it seems probable that, as in other parts of the brain (Garthwaite 1993), the actions of Nitric Oxide may be mediated via the guanylate cyclase system (Shaw et al, 1999).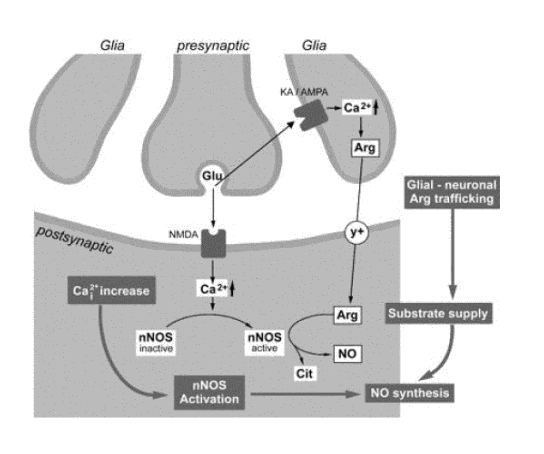
Schematic representation of the proposed model of co-occurrence of the glial-neuronal arginine (Arg) transfer and nNOS (nitric oxide synthase) activation. Glutamate (Glu) evokes the release of Arg from glia via stimulation of glial inotropic non-NMDA receptors. Arg is transported to NOS containing neurons via the y+ uptake system. Our data show that Arg availability, which depends on the activation of ionotropic non-NMDA receptors, determines the rate of NO biosynthesis by neuronal NOS. Moreover, Glu, through stimulation of postsynaptic NMDA receptors and Ca2+ increase, can activate neuronal NOS. NO formation may, thus, depend on the coincident occurrence of the glial-neuronal transfer of Arg and NOS activation (Do et.al., 2003).
This page was written by Tom Salt, and is part of the Neurotransmitters in Sensory Systems Home Page.
 |