Contributions of mGlu1 and mGlu5 receptors to interactions with N-methyl-D-aspartate receptor-mediated responses and nociceptive sensory responses of rat thalamic neurones.
T E Salt and K E Binns
Institute of Ophthalmology, University
College London, 11-43 Bath Street, LONDON EC1V 9EL
Keywords
metabotropic glutamate receptors; ventrobasal thalamus; pain; NMDA receptors;
in vivo electrophysiology; somatosensory synapses
ABSTRACT
Previous work from this laboratory has
shown that nociceptive responses of rat ventrobasal thalamus neurones can
be reduced by N-methyl-D-aspartate (NMDA) antagonists and by selective
metabotropic glutamate receptor mGlu1 antagonists. The recent development
of the mGlu5-selective antagonist 6-methyl-2-(phenylethynyl)-pyridine (MPEP)
now allows the direct probing of possible mGlu5 involvement in thalamic
nociceptive responses. Extracellular recordings
were made from single neurones in the ventrobasal thalamus and immediately
overlying dorsal thalamic nuclei of adult urethane-anaesthetised rats using
multi-barrel electrodes. Responses
of neurones to iontophoretic applications of the mGlu5-selective agonist
(R,S)-2-chloro-5-hydroxyphenylglycine (CHPG) were selectively reduced during
continuous iontophoretic applications of MPEP. Similar applications of
MPEP reduced neuronal responses to noxious thermal stimuli to 53±9.5
% of control responses. Co-application by iontophoresis of NMDA
and metabotropic glutamate receptor agonists resulted in a mutual potentiation
of excitatory responses. This effect could be reduced by either MPEP or
the mGlu1 antagonist LY367385. These results, taken together with previous
data, suggest that acute thalamic nociceptive responses are mediated by
a combination of mGlu1, mGlu5 and NMDA receptor activation, and that co-activation
of these receptors produces a synergistic excitatory effect. Thus blockade
of any of these receptor types would have a profound effect on the
overall nociceptive response.
INTRODUCTION
It is known that metabotropic glutamate receptors can play a role in
the signalling of nociceptive information both spinally and supra-spinally13,15,17,27,28,40,45.
The mGlu receptors can be divided into three groups, I-III, on the basis
of their sequence homologies, their agonist and antagonist pharmacology,
and their coupling to intracellular transduction mechanisms in expression
systems10,25. The Group I (mGlu1 and mGlu5) receptors, which
are known to couple to postsynaptic inositol phosphate metabolism, have
in particular been implicated in nociceptive responses in both the spinal
cord and the thalamus8,13,15,17,28,40,45. This is based on the
use of antagonists with varying degrees of selectivity, and upon the use
of antisense oligonucleotides. Some of these studies have suggested a role
for mGlu1 receptors at both the level of the spinal cord45 and
the thalamus40. However, the demonstration of a specific role
for mGlu5 receptors in nociception has not been possible until recently
due to the lack of specific antagonists for this receptor. The development
of the selective mGlu5 antagonist MPEP has provided a suitable pharmacological
tool for experimental purposes18,37.
The ventrobasal (VB) thalamus is a pivotal relay and processing point
for somatosensory information ascending from the spinal cord to the cerebral
cortex, and as such it is clearly a prime potential site for the action
of analgesic drugs21,30. We have previously shown that mGlu1
receptors40 and NMDA receptors14 contribute to nociceptive
responses of rat thalamic neurones. The aim of the present study was to
determine whether a role in acute nociceptive signalling could be identified
for mGlu5 receptors in addition to mGlu1 receptors in the thalamus by the
use of MPEP. Furthermore, in view of the known interactions between Group
I mGlu receptors and NMDA receptors in several brain areas2,12,16,20,31,
and the involvement of NMDA receptors in thalamic nociceptive processing4,14,
it was of interest to determine whether functional interactions between
NMDA responses and mGlu1 and mGlu5 responses could occur in the thalamus
in vivo. We have attempted to investigate this using a number of agonists
which show activity at Group I mGlu receptors and the selective mGlu1 and
mGlu5 antagonists LY3673859 and MPEP18. Some of these
results have been presented in abstract form.35,36
METHODS
Experiments were carried out in adult Wistar rats (300-500g) anaesthetised
with urethane (1.2g/kg. I.P.), as previously described 34. A
tracheal cannulation was made and the rats were allowed to breathe spontaneously.
In some animals an external jugular vein was cannulated to allow intravenous
administration of drugs. The electrocardiogram waveform and rate was monitored
throughout each experiment via limb surface electrodes. The electroencephalogram
was recorded and monitored throughout the experiment via two screw electrodes
fixed and cemented over the frontal-occipital cortex contralateral to the
thalamus from which single neurone recordings were made. Anaesthesia was
periodically supplemented during the experiment (50mg urethane, I.P. bolus)
as necessary to maintain absence of withdrawal reflexes to hind paw pressure.
All animal experiments were carried out in accordance with the U.K. Animals
(Scientific Procedures) Act, 1986 and associated guidelines.
Extracellular recordings were made from single neurones in the VB thalamus
and immediately dorsal thalamus using the central barrel of seven-barrel
iontophoretic micropipettes. Action potential spikes were gated using a
hardware spike-discriminator whose output pulses were timed and recorded
by the CED1401 interface and computer system. The amplitude and shape of
the gated action potentials were monitored throughout the recording session.
Neurones were identified on the basis of their stereotaxic location and
their responses to somatosensory (nociceptive and non-nociceptive) stimuli,
as described previously 19,30,34. Iontophoretic applications
of glutamate receptor agonists and antagonists were made from the outer
barrels of the micropipettes which contained one of the following aqueous
solutions: ACPD [(1S,3R)-1-aminocyclopentane-1,3-dicarboxylate] 50mM, pH8;
CHPG [(R,S)-2-Chloro-5-hydroxyphenylglycine] 100mM, pH8; DHPG [(S)-3,5-Dihydroxyphenylglycine]
50mM, pH5; NMDA [N-methyl-D-aspartate] 50mM, pH8; AMPA [(R,S)-a-amino-3-hydroxy-5-methyl-4-isoxazolepropionate]
50mM, pH8; MPEP [6-methyl-2-(phenylethynyl)-pyridine] 2mM or 10mM in 150mM
NaCl, pH5; LY367385 [(+)-2-methyl-4-carboxyphenylglycine]
50mM, pH8. In addition, one barrel contained 1M NaCl for automatic
current balancing. All drugs were ejected iontophoretically as anions (with
the exception of MPEP and DHPG), and prevented from diffusing out of the
pipette by a retaining current (10-20nA) of opposite polarity to the ejection
current. MPEP was provided by Novartis (Basel, Switzerland), LY367385 by
Lilly Research Labs (Erl Wood Manor, UK), other drugs were purchased from
Tocris (Bristol, UK) or Sigma.
Regular repeated cycles of agonist ejections were set up and initiated
by a computer system, and extracellular action potentials were gated and
timed using the computer system, which could produce peristimulus-histograms
of single-neurone activity. Agonist ejection
parameters were adjusted so as to produce sub-maximal responses. In the
experiments where interactions between agonists were investigated, agonist
ejections were adjusted to produce only minimal responses when ejected
alone. The effects of antagonists were assessed by continuous iontophoretic
application of antagonists during several cycles of agonist ejection. Antagonist
ejection currents and durations were adjusted so as to achieve selective
antagonism of appropriate agonist responses compared to responses to other
agonists of the same neurones.
Nociceptive responses were evoked by immersion
of part of either the contralateral hindpaw or the tail in water of 52C
for 20-30sec. These stimuli were repeated at 5 minute intervals. Responses
to such stimuli typically increased during the course of the stimulus and
outlasted the stimulus by up to two minutes, as described previously13,14,29.
When nociceptive responses were challenged with iontophoretic application
of an antagonist, the duration and magnitude of the iontophoresis current
was in all cases the same as that which had been shown to be effective
and selective against the appropriate agonist on the same neurone.
Responses to agonists or noxious stimuli were quantified as the number
of action potentials evoked by agonist ejection or stimulus. The effects
of antagonists on these responses were assessed by calculating the agonist
or stimulus response during antagonist application as a percentage of the
response under control conditions. In agonist interaction experiments,
the number of action potentials evoked by the co-application of agonists
and by the application of the iGlu agonist alone and the mGlu agonist alone
were counted and used to calculate an estimate of the degree of response
potentiation as shown below:
The potentiation excess (Px) = [number of action potentials obtained ]
Where the number of action potentials obtained was equal to the response to the coapplication of agonists (minus background activity) and the number of action potentials expected was equal to the sum of the response to the iGlu agonist (minus background activity) and the response to the mGlu agonist (minus background activity).minus [number of action potentials expected]
Data from individual neurones were used to compute mean values of effects.
Statistical comparisons of these values under control conditions and during
antagonist applications were made using the Wilcoxon Signed Rank test.
Results were deemed to be significant when P<0.05 .
RESULTS
Metabotropic Glutamate Receptors and nociceptive responses.
The effects of the mGlu5 antagonist MPEP applied iontophoretically on
responses to the mGlu5-selective agonist CHPG12, the broad-spectrum
mGlu agonist ACPD10, and NMDA were investigated in 17 neurones.
In all of these neurones responses to regular ejections of agonists were
recorded over several 5-minute cycles prior to the ejection of MPEP. The
antagonist was then continuously ejected for one or more agonist ejection
cycles, and the MPEP ejection was terminated when a selective effect was
seen or it was deemed that no effect was evident. Agonist ejection cycles
were then continued until recovery from the effects of MPEP were seen (5-15
minutes). MPEP ejection currents and durations were adjusted so as to produce
selective antagonism of CHPG responses whilst having little or no effect
on NMDA responses. Under these conditions MPEP (20-120 nA) reduced responses
to CHPG to32 % of control whilst having less effect on ACPD responses and
no significant effect on NMDA responses (Table 1a). Six of these neurones
were then studied further with noxious stimuli: the agonist ejection cycles
were terminated and replaced with noxious stimuli applied at 5 minute intervals.
When control responses to such stimuli had been established, MPEP was applied
continously with the same ejection current and duration which had been
found to be effective against CHPG on that neurone. Under these conditions
MPEP was able to reduce the responses to noxious stimulation to an average
of 53% of control levels (Table 1b, Figure 1). We have previously shown
that the mGlu1-selective antagonist LY367385 9 can reduce responses
of thalamic neurones to ACPD compared to CHPG and reduce responses to noxious
stimuli to 51% of control values 40,41. These data are therefore
included for comparison (Table 2).
Interactions between N-methyl-D-aspartate and Metabotropic Glutamate
Receptor agonists.
In order to investigate the possibility for mutual enhancement of responses
mediated by NMDA receptors and those mediated by Group I mGlu receptors,
experiments were carried out where responses of thalamic neurones to NMDA
and mGlu agonists alone were compared with those where the two types of
agonist were applied together. In such experiments, responses to the two
agonists applied together were greater than the arithmetic sum of the responses
to the agonists when applied alone (Table 3). This was the case when NMDA
was co-applied with any of CHPG, ACPD or DHPG (Figure 2). In some experiments,
either MPEP or LY367385 was applied during this protocol. MPEP was able
to reduce the effects of CHPG application, whereas LY367385 was able to
reduce the effects of ACPD application (Figure 2 and Table 4). In order
to investigate the selectivity of the interaction between NMDA and mGlu
agonists, some experiments were also carried out with the ionotropic agonist
AMPA and ACPD. It was found that there was a potentiation of responses
when these agonists were co-applied, similar to that seen when NMDA and
mGlu receptor agonists were co-applied (Table 3).
DISCUSSION
In the present study we have found that the mGlu5 antagonist MPEP reduces
nociceptive responses of thalamic neurones. In addition, we have confirmed
the previous findings by ourselves37 and others18
that MPEP is a suitable antagonist to block mGlu5 receptor-mediated responses
in the brain. It is known that mGlu5 receptors are present in the rat thalamus1,26,32,43,
and it is likely that these receptors are responsible for the excitatory
responses seen upon iontophoretic application of CHPG, which are antagonised
by iontophoretically-applied MPEP37. Interestingly, responses
to the mixed mGlu-receptor agonist ACPD were much less affected by MPEP,
indicating that these responses are mediated predominantly by a receptor
other than mGlu5. Given the comparatively high levels of mGlu1 receptors
in the thalamus23,26,42, it is probable that the major receptor
contribution to the ACPD responses is via mGlu1. This is supported by our
previous finding that thalamic responses to ACPD are very sensitive to
the selective mGlu1 receptor antagonist LY36738541. It is also
of interest that responses to CHPG were not completely blocked by MPEP
(Table 1A) and that LY367385 in fact had a small effect on such responses
(Table 2A). This suggests that CHPG may have some effect at mGlu1 receptors.
In this study, we have shown that application of either ACPD, DHPG or
CHPG can potentiate responses to NMDA. Furthermore, these effects can be
reduced by the selective antagonists LY367385 and MPEP. This suggests that
activation of either mGlu1 or mGlu5 receptors results in potentiation
of NMDA receptor-mediated responses. This is consistent with other reports
in the literature which indicate that there is an interaction between NMDA
and Group I mGlu receptors2,12,16,20,31. However, it is also
evident from our data that an interaction between AMPA and mGlu responses
is possible in the thalamus, an effect also seen in other brain areas3,6,7,11,22.
It is known that activation of Group I mGlu receptors in the thalamus in
vitro causes a postsynaptic depolarisation associated with a increase
in input resistance, possibly due to a reduction in a membrane potassium
conductance24,44. Such an effect would result in the potentiation
of other depolarising inputs mediated by an inward current, such as those
due to NMDA or AMPA receptor activation. It is thus possible that the potentiation
between NMDA/AMPA and mGlu responses seen in the present study is due to
such a mechanism, rather than a specific interaction at the receptor level,
or that the potentiation seen is a combination of these factors3,22.
This would require further investigation. Nevertheless, from a functional
point of view, there is clearly a powerful facilitatory interaction between
iGlu and mGlu1/mGlu5 receptor activation in the thalamus in vivo.
We have shown previously that nociceptive responses of thalamic neurones
can be reduced by broad-spectrum mGlu antagonists13,38 and by
the mGlu1-selective antagonist LY36738540, as well as by NMDA
antagonists14. In this study we have now shown that thalamic
nociceptive responses are sensitive to an mGlu5 antagonist. Interestingly,
blockade of mGlu receptors does not result in a reduction of non-nociceptive
(vibrissa/hair follicle afferent) responses in the thalamus13,
although NMDA receptor blockade does reduce such responses33.
Taken together with the present findings, this indicates that there is
a role for mGlu1, mGlu5 and NMDA receptors in the generation of thalamic
nociceptive responses. It is intriguing that when broad-spectrum mGlu antagonists
were used,13 a slightly greater reduction of nociceptive responses
was seen than with the subtype-selective antagonists MPEP or LY367385.
This further suggests that there is activation of both mGlu1 and mGlu5
and that these have distinct contributions to the nociceptive response.
Furthermore, the potent effect of MPEP against nociceptive responses suggests
that mGlu5 is an important contributor to supraspinal nociception even
though mGlu1 is the predominant receptor in the thalamus. The current experiments
indicate a role for Group I mGlu receptors in acute nociception. However,
it is also evident that such receptors participate in chronic nociception
and central sensitisation5,27, a matter that will need to be
addressed for thalamic responses in the future.
We have now shown that activation of mGlu1 and/or mGlu5 receptors can
potentiate responses to NMDA in the thalamus and it is thus likely that
these receptors interact synergistically to produce the nociceptive response
in the thalamus. A consequence of this would be that blockade of any
of these receptor types would have a profound effect on the overall nociceptive
response. Furthermore, given the likely complexities of mGlu receptor interactions
with presynaptic and postsynaptic, and excitatory and inhibitory synaptic
elements in the thalamus 39, it is probable that there are still
further factors to be elucidated in this system.
Acknowledgements
We thank F Gasparini & R Kuhn for donations of MPEP and B Clark & A Kingston for donations of LY367385. This study was supported by Novartis and The Wellcome Trust.
| MPEP | Nociceptive | CHPG | ACPD | NMDA | n |
| A | 32±3.3** | 85±7.8* | 101±8.7 | 17 | |
| B | 53±9.5* | 29±5.3* | 83±9.0 | 101±8.9 | 6 |
A. Data for all neurones where MPEP was tested. B.
Data from a sub-set of neurones where sensitivity of nociceptive responses
to MPEP was also tested. Values in the table for each nociceptive or agonist
response type are means of percentage of control + SEM for n
neurones. Values marked with * or ** are significantly different from control
values (P<0.05, P<0.01: Wilcoxon signed rank test).
Table 2. Effects of LY367385
| LY367385 | Nociceptive | CHPG | ACPD | NMDA | n |
| A | 78±9.1* | 11±2.4** | 128±10.3* | 9 | |
| B | 51±5.8** | 17±3.9** | 110±8.6 | 10 |
A. Data for neurones where LY367385 was tested against
ACPD and CHPG41. B. Data from neurones where sensitivity
of nociceptive responses to LY367385 was tested40. Values in
the table for each nociceptive or agonist response type are means of percentage
of control + SEM for n neurones. Values marked with * or
** are significantly different from control values (P<0.05, P<0.01:
Wilcoxon signed rank test).
Table 3. Potentiation of the effects of NMDA and AMPA by co-application
with Group I mGlu receptor agonists.
| Co-applied agonists | response expected
(action potentials) |
response obtained
(action potentials) |
Px | n |
| NMDA / ACPD | 112 ± 23.7 | 419 ± 51.5** | 307 ± 37.2 | 16 |
| NMDA / CHPG | 106 ± 12.9 | 325 ± 48.5** | 218 ± 46.2 | 16 |
| NMDA / DHPG | 117 ± 42.4 | 269 ± 46.4* | 152 ± 23.6 | 6 |
| AMPA / ACPD | 119 ± 45.2 | 513 ± 126.5** | 393 ± 90.7 | 7 |
Comparisons of the response obtained with the response expected upon
co-application of different iGlu and mGlu agonists, and the calculated
Potentiation excess (Px) value. Values in the table are means +
SEM for n neurones. Values marked with * or ** are significantly
different from expected values (P<0.05, P<0.01: Wilcoxon signed rank
test).
Table 4. Effects of antagonists on agonist potentiation.
| Co-applied agonists/antagonist | response expected
(action potentials) |
response obtained
(action potentials) |
Px | n |
| NMDA / ACPD | 85 ± 21.5 | 428 ± 66.2 | 343 ± 57.0 | 6 |
| NMDA / ACPD
with LY367385 |
70 ± 19.2 | 184 ± 42.9 | 114 ± 33.3* | |
| NMDA / CHPG | 104 ± 25.1 | 288 ± 65.4 | 184 ± 44.2 | 5 |
| NMDA / CHPG
with MPEP |
138 ± 55.4 | 79 ± 20.4 | 13 ± 35.6* |
Similar values to those shown in Table 3, but taken from those experiments where either LY367385 or MPEP were applied. Note that the antagonists significantly reduced the Px values (* P<0.05: Wilcoxon signed rank test).
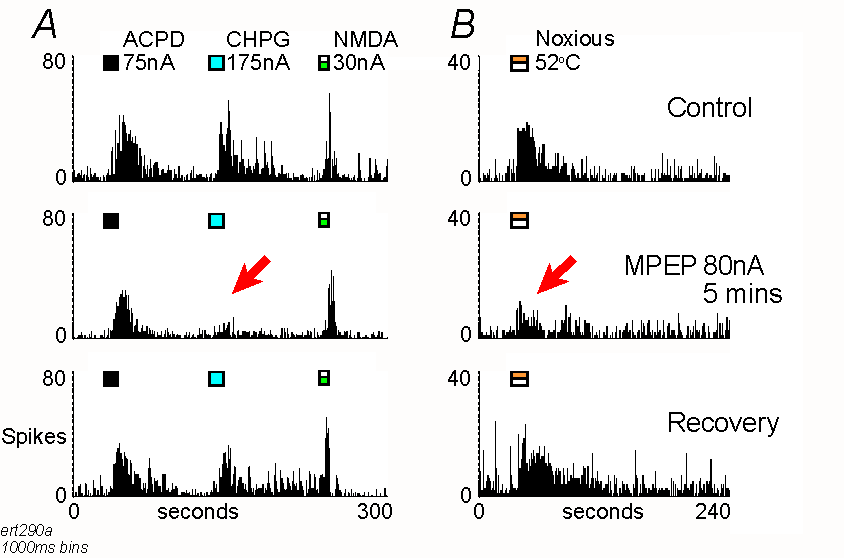
Responses of a single thalamic neurone to iontophoretic applications
of ACPD, CHPG and NMDA (A) and noxious thermal stimulation
(B). Records are peristimulus-histograms of action potentials
("spikes") collected in successive 1000ms epochs ("bins") under either
control conditions, during MPEP iontophoresis, or recovery after the end
of the MPEP ejection. Timing of agonist ejections and stimulation are shown
by the marker bars. Note that MPEP selectively reduced responses to CHPG
(A) and noxious stimulation (B), as indicated
by the arrows.

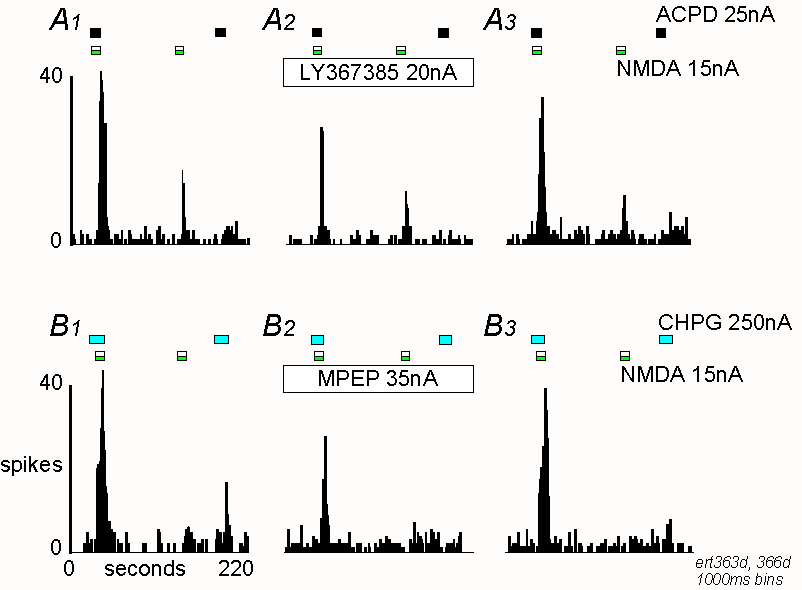
Peristimulus time histograms showing potentiation between ACPD and NMDA
(A) and CHPG and NMDA (B) taken from two different
neurones. For details see figure 1.
A1 Responses to ACPD and NMDA alone and together under
control conditions. Note the potentiation of responses when the agonists
are co-applied. A2 Responses during the continuous application
of LY367385, 15 minutes after the start of the antagonist ejection. A3
Recovery from the effects of LY367385.
B1-B3 Similar records showing potentiation between CHPG
and NMDA, and the effect of MPEP, 10 minutes after the start of the antagonist
ejection.
1. Abe T., Sugihara H., Nawa H., Shigemoto R., Mizuno N., and Nakanishi S. (1992) Molecular characterization of a novel metabotropic glutamate receptor mGluR5 coupled to inositol phosphate/Ca2+ signal transduction. J. Biol. Chem. 267, 13361-13368.
2. Alagarsamy S., Marino M.J., Rouse S.T., Gereau R.W., Heinemann S.F., and Conn P.J. (1999) Activation of NMDA Receptors Reverses Desensitization of mGluR5 in Native and Recombinant Systems. Nature Neurosci. 2, 234-240.
3. Bond A. and Lodge D. (1995) Pharmacology of metabotropic glutamate receptor-mediated enhancement of responses to excitatory and inhibitory amino acids on rat spinal neurones in vivo. Neuropharmacol. 34, 1015-1023.
4. Bordi F. and Quartaroli M. (2000) Modulation of Nociceptive Transmission by NMDA/Glycine Site Receptor in the Ventroposterolateral Nucleus of the Thalamus. Pain 84, 213-224.
5. Bowes M., Panesar M., Gentry C., Urban L., Gasparini F., Kuhn R., and Walker K. (1999) Anti-hyperalgesic effects of the novel metabotropic glutamate receptor 5 antagonist Methylphenylethynlpyridine, in rat models of inflammatory pain. Brit. J. Pharmacol. 126, 250P.
6. Calabresi P., Centonze D., Gubellini P., Marfia G.A., and Bernardi G. (1999) Glutamate-Triggered Events Inducing Corticostriatal Long-Term Depression. J. Neurosci. 19, 6102-6110.
7. Cerne R. and Randic M. (1992) Modulation of AMPA and NMDA responses in rat spinal dorsal horn neurons by trans-1-aminocyclopentane-1,3-dicarboxylic acid. Neurosci. Lett. 144, 180-184.
8. Chen Y., Bacon G., Sher E., Clark B.P., Kallman M.J., Wright R.A., Johnson B.G., Schoepp D.D., and Kingston A.E. (2000) Evaluation of the Activity of a Novel Metabotropic Glutamate Receptor Antagonist (±)-2-Amino-2-(3-Cis and Trans-Carboxycyclobutyl-3-(9-Thioxanthyl Acid) in the in Vitro Neonatal Spinal Cord and in an in Vivo Pain Model. Neurosci. 95, 787-793.
9. Clark B.P., Baker S.R., Goldsworthy J., Harris J.R., and Kingston A.E. (1997) (+)-2-Methyl-4-carboxyphenylglycine (LY367385) selectively antagonises metabotropic glutamate mGluR1 receptors. Bioorg. & Med. Chem. Lett. 7, 2777-2870.
10. Conn P.J. and Pin J.P. (1997) Pharmacology and functions of metabotropic glutamate receptors. Ann. Rev. Pharmacol. Toxicol. 37, 207-237.
11. Dev K.K. and Henley J.M. (1998) The Regulation of AMPA Receptor-Binding Sites. Mol. Neurobiol. 17, 33-58.
12. Doherty A.J., Palmer M.J., Henley J.M., Collingridge G.L., and Jane D.E. (1997) (R,S)-2-chloro-5-hydroxyphenylglycine (CHPG) activates mGlu5 but not mGlu1 receptors expressed in CHO cells and potentiates NMDA responses in the hippocampus. Neuropharmacol. 36, 265-267.
13. Eaton S.A., Birse E.F., Wharton B., Sunter D.C., Udvarhelyi P.M., Watkins J.C., and Salt T.E. (1993) Mediation of thalamic sensory responses in vivo by ACPD-activated excitatory amino acid receptors. Eur. J. Neurosci. 5, 186-189.
14. Eaton S.A. and Salt T.E. (1990) Thalamic NMDA receptors and nociceptive sensory synaptic transmission. Neurosci. Lett. 110, 297-302.
15. Fisher K. and Coderre T.J. (1996) The Contribution of Metabotropic Glutamate Receptors (mGluRs) to Formalin-Induced Nociception. Pain 68, 255-263.
16. Fitzjohn S.M., Irving A.J., Palmer M.J., Harvey J., Lodge D., and Collingridge G.L. (1996) Activation of group I mGluRs potentiates NMDA responses in rat hippocampal slices. Neurosci. Lett. 203, 211-213.
17. Fundytus M.E., Fisher K., Dray A., Henry J.L., and Coderre T.J. (1998) In Vivo Antinociceptive Activity of Anti-Rat mGluR(1) and mGluR(5) Antibodies in Rats. Neuroreport 9, 731-735.
18. Gasparini F., Lingenhohl K., Stoehr N., Flor P.J., Heinrich M., Vranesic I., Biollaz M., Allgeier H., Heckendorn R., Urwyler S., Varney M.A., Johnson E.C., Hess S.D., Rao S.P., Sacaan A.I., Santori E.M., Velicelebi G., and Kuhn R. (1999) 2-Methyl-6-(Phenylethynyl)-Pyridine (MPEP), a Potent, Selective and Systemically Active mGlu5 Receptor Antagonist. Neuropharmacol. 38, 1493-1503.
19. Guilbaud G., Peschanski M., Gautron M., and Binder D. (1980) Neurones responding to noxious stimulation in VB complex and caudal adjacent regions in the thalamus of the rat. Pain 8, 303-318.
20. Holohean A.M., Hackman J.C., and Davidoff R.A. (1999) Mechanisms Involved in the Metabotropic Glutamate Receptor-Enhancement of NMDA-Mediated Motoneurone Responses in Frog Spinal Cord. Brit. J. Pharmacol. 126, 333-341.
21. Jones E.G. (1985) The Thalamus. Plenum Press, New York.
22. Jones M.W. and Headley P.M. (1995) Interactions between metabotropic and ionotropic glutamate receptor agonists in the rat spinal cord in vivo. Neuropharmacol. 34, 1025-1031.
23. Martin L.J., Blackstone C.D., Huganir R.L., and Price D.L. (1992) Cellular localization of a metabotropic glutamate receptor in rat brain. Neuron 9, 259-270.
24. McCormick D.A. and Von Krosigk M. (1992) Corticothalamic activation modulates thalamic firing through glutamate "metabotropic" receptors. Proc. Nat. Acad. Sci. (USA) 89, 2774-2778.
25. Nakanishi S. (1992) Molecular diversity of glutamate receptors and implications for brain function. Science 258, 597-603.
26. Neto F.L., Schadrack J., Berthele A., Zieglgansberger W., Tolle T.R., and Castro-Lopes J.M. (2000) Differential distribution of metabotropic glutamate receptor subtype mRNAs in the thalamus of the rat. Brain Res. 854, 93-105.
27. Neugebauer V., Chen P.S., and Willis W.D. (1999) Role of Metabotropic Glutamate Receptor Subtype Mglur1 in Brief Nociception and Central Sensitization of Primate Stt Cells. J. Neurophysiol. 82, 272-282.
28. Neugebauer V, Luecke T, and Schaible H-G (1994) Requirement of metabotropic glutamate receptors for the generation of inflammation-evoked hyperexcitability in rat spinal cord neurons. Eur. J. Neurosci. 6, 1179-1186.
29. Peschanski M., Guilbaud G., Gautron M., and Besson J.M. (1980) Encoding of noxious heat messages in neurons of the ventrobasal thalamic complex of the rat. Brain Res. 197, 401-413.
30. Peschanski M., Guilbaud G., Lee C.L., and Mantyh P.W. (1983) Involvement of the rat ventrobasal thalamic complex in the sensory-discriminative aspects of pain: electrophysiological and anatomical data. In Somatosensory Integration in the Thalamus (ed. Macchi G, Rustioni A, and Spreafico R), pp. 147-163. Elsevier Science Publishers, Amsterdam.
31. Pisani A., Calabresi P., Centonze D., and Bernardi G. (1997) Enhancement of NMDA responses by group I metabotropic glutamate receptor activation in striatal neurones. Brit. J. Pharmacol. 120, 1007-1114.
32. Romano C., Van den Pol A.N., and O'Malley K.L. (1996) Enhanced early developmental expression of the metabotropic glutamate receptor mGluR5 in rat brain: Protein, mRNA splice variants, and regional distribution. J. Comp. Neurol. 367, 403-412.
33. Salt T.E. (1986) Mediation of thalamic sensory input by both NMDA and non-NMDA receptors. Nature 322, 263-265.
34. Salt T.E. (1987) Excitatory amino acid receptors and synaptic transmission in the rat ventrobasal thalamus. J. Physiol. 391, 499-510.
35. Salt T.E., Binns K.E., and Turner J.P. (1999) Antagonism of thalamic metabotropic glutamate (mGlu) receptor and nociceptive responses by the novel mGlu5 antagonist MPEP. Society for Neurosci., Abstracts 25, 371.14.
36. Salt T.E., Binns K.E., and Turner J.P. (1999) Antagonism of Group I Metabotropic Glutamate Receptor and Sensory Responses by Novel Selective mGlu5 and mGlu1 Antagonists in the Thalamus. Neuropharmacol. 38, 123.
37. Salt T.E., Binns K.E., Turner J.P., Gasparini F., and Kuhn R. (1999) Antagonism of the mGlu5 agonist 2-chloro-5-hydroxyphenylglycine by the novel selective mGlu5 antagonist 6-methyl-2-(phenylethynyl)-pyridine (MPEP) in the thalamus. Brit. J. Pharmacol. 127, 1057-1059.
38. Salt T.E. and Eaton S.A. (1994) The function of metabotropic excitatory amino acid receptors in synaptic transmission in the thalamus: studies with novel phenylglycine antagonists. Neurochem. Int. 24, 451-458.
39. Salt T.E. and Eaton S.A. (1996) Functions of ionotropic and metabotropic glutamate receptors in sensory transmission in the mammalian thalamus. Prog. Neurobiol. 48, 55-72.
40. Salt T.E. and Turner J.P. (1998) Reduction of sensory and metabotropic glutamate receptor responses in the thalamus by the novel mGluR1selective antagonist (S) 2-methyl-4-carboxy-phenylglycine. Neurosci. 85, 655-658.
41. Salt T.E., Turner J.P., and Kingston A.E. (1999) Evaluation of agonists and antagonists acting at Group I metabotropic glutamate receptors in the thalamus in vivo. Neuropharmacol. 38, 1505-1510.
42. Shigemoto R., Nakanishi S., and Mizuno N. (1992) Distribution of the mRNA for a metabotropic glutamate receptor (mGluR1) in the central nervous system: An in situ hybridization study in adult and developing rat. J. Comp. Neurol. 322, 121-135.
43. Shigemoto R, Nomura S, Ohishi H, Sugihara H, Nakanishi S, and Mizuno N (1993) Immunohistochemical localization of a metabotropic glutamate receptor, mGluR5, in the rat brain. Neurosci. Lett. 163, 53-57.
44. Turner J.P. and Salt T.E. (1998) Characterization of sensory and corticothalamic excitatory inputs to rat thalamocortical neurones in vitro. J. Physiol. 510, 829-843.
45. Young M.R., Blackburn-Munro G., Dickinson T., Johnson M.J., Anderson H., Nakalembe I., and Fleetwood-Walker S.M. (1998) Antisense ablation of type I metabotropic glutamate receptor mGluR(1) inhibits spinal nociceptive transmission. J. Neurosci. 18, 10180-10188.
ACPD (1S,3R)-1-aminocyclopentane-1,3-dicarboxylate
AMPA (R,S)-a-amino-3-hydroxy-5-methyl-4-isoxazolepropionate
CHPG (R,S)-2-Chloro-5-hydroxyphenylglycine
DHPG (S)-3,5-Dihydroxyphenylglycine
iGlu ionotropic glutamate
LY367385 (+)-2-methyl-4-carboxyphenylglycine
MPEP 6-methyl-2-(phenylethynyl)-pyridine
mGlu metabotropic glutamate
NMDA N-methyl-D-aspartate
VB ventrobasal