11-43 Bath Street, LONDON EC1V 9EL.
Single-neurone recordings were made with extracellular multi-barrel iontophoretic electrodes in
the ventrobasal thalamus and immediately overlying dorsal thalamic nuclei of adult male Wistar
rats anaesthetised with urethane (1.2 g/kg, I.P.), as detailed previously21. The outer barrels were
used for iontophoretic drug applications, and each contained one of the following substances, as
Na+ salts: NMDA [N-methyl-D-aspartate], AMPA [(±)-a-amino-3-hydroxy
-5-methyl-4-isoxazolepropionate], ACPD [1S,3R-aminocyclopentane-1,3-dicarboxylate],
LY367385 [S-2-methyl-4-carboxy-phenylglycine] (all 50mM in water, pH 8.0-8.5), DHPG
[S-3,5-dihydroxyphenylglycine] (50mM in water, pH 5.5) and 1M NaCl (for current balancing),
and Pontamine Sky Blue dye (2.5% in 0.5M NaCl/0.5M Na acetate). All drugs were ejected
iontophoretically as anions (with the exception of DHPG), and prevented from diffusing out of
the pipette by a retaining current (10-20nA) of opposite polarity to the ejection current. All drugs
were obtained from Tocris, apart from LY367385 (gift from Lilly Research). Regular repeated
cycles (5 minute duration) of agonist ejections were set up and initiated by a computer system,
and extracellular action potentials were gated and timed using the computer system, which could
produce peristimulus-histograms of single-neurone activity.
Responses of neurones to iontophoretic applications of either ACPD (18 neurones) or DHPG (4
neurones) were significantly reduced during continuous iontophoretic application of LY367385
(10-40nA) whereas responses to either N-methyl-D-aspartate (NMDA) or (AMPA) were
relatively unaffected (Table 1). The effects of the antagonist were seen from between 5 and 15
minutes after the start of the iontophoretic ejections, and lasted for between 5 and 20 minutes
after the end of the ejections (Figure 1A). Given that LY367385 appeared to be a selective
antagonist, we studied the effects this compound on the nociceptive responses of ten of these
neurones. Nociceptive responses were evoked by immersion of part of either the contralateral
hindpaw or the tail in water of 52oC for 15-25sec. These stimuli were repeated at 5 minute
intervals. Responses to such stimuli typically increased during the course of the stimulus and
outlasted the stimulus by up to two minutes, as described previously5,7,17. Application of
LY367385 with the same iontophoretic currents and ejection durations which had produced
selective antagonism on the same neurones was found to reduce the nociceptive responses of all
of these neurones to 51±5.8% of their control responses (Figure 1B, Table 1). In addition, a
further six of the 22 neurones recorded were studied to investigate the effects of LY367385 on
non-nociceptive responses evoked by stimulation of the vibrissae with an air jet (2 second
duration)21. The antagonist had little effect on these responses (101±8.1% of control responses),
although responses of the same neurones to either ACPD or DHPG were reduced by LY367385.
The data obtained with LY367385 are consistent with our previous findings with a number of
phenylglycine antagonists in the thalamus 5,22. Given the high selectivity of LY367385 for
mGluR1 over mGluR54, the predominant expression of mRNA for mGluR1 compared to mGluR5
in the thalamus1,12,20,24, and the immunohistochemical staining for mGluR1a rather than mGluR5
on the thalamic relay neurone dendrites9,11,26, it seems highly likely that the ability of LY367385 to
antagonise ACPD and DHPG responses in the present study is attributable to an action at
mGluR1. Thus, this compound appears to be a useful tool to distinguish between mGluR1 and
mGluR5 in studies of synaptic function. In this study, we have exploited this in order to test more
directly whether sensory responses of thalamic neurones involve mGluR1: our finding that
LY367385 reduces thalamic nociceptive responses now provides strong evidence that these
responses are mediated in part by mGluR1. It is of course possible that other mGluRs may also
contribute to this response, but more conclusive data cannot be obtained until appropriate
selective antagonists (e.g. for mGluR5) are developed. The reduction of responses to nociceptive
stimuli by LY367385 is the first direct pharmacological demonstration of a synaptic response
mediated by mGluR1.
The anatomical localisation of mGluR1a in the thalamus suggests that it may be postsynaptic to
cortical axon terminals9,11,26. Furthermore, there is some electrophysiological evidence to suggest
that the corticothalamic input may utilise Group I mGluRs6,14. This raises the intriguing possibility
that the nociceptive response of thalamic neurones may be dependent upon a cortical input which
may be mediated, at least partly, via mGluR1. It is of interest to note that we have previously
found that thalamic nociceptive responses also show an involvement of NMDA receptors7. Thus
it is likely that the complete physiological response to such stimuli is a summation of NMDA
receptor and mGluR1 components (possibly with other components still to be identified). It is
well-known that NMDA receptor mediated responses are voltage-dependent under physiological
Mg++ concentrations13,16, and that postsynaptic Group I mGluR responses may be mediated via a
decreased K+ conductance3,14,25. Thus, an mGluR input would be in a good position to amplify the
non-linear NMDA-receptor mediated response: this would make a combined
NMDA-receptor/mGluR1 input an effective signalling system. This could be further enhanced by
the second-messenger-mediated facilitation of NMDA responses by Group I mGluRs which is
known in several systems2,8,19. This could also provide a mechanism for long-term changes in
response to noxious inputs, which may be important in the development of central pain
syndromes5.
In conclusion, we have shown that the novel antagonist LY367385 is a useful tool for studies of
synaptic pharmacology aimed at identifying a role for mGluR1. We have used this compound to
show that physiological responses to noxious stimuli of thalamic neurones are partly mediated by
mGluR1. This finding may be of importance in the development of novel analgesic therapies.
This work was supported by the Wellcome Trust. We are grateful to Dr A E Kingston, Lilly
Research Centre, Windlesham, for the gift of LY367385 and helpful discussions.
| ACPD
(% control) |
DHPG
(% control) |
NMDA
(% control) |
AMPA
(% control) |
Nociceptive
(% control) |
Non-Nociceptive
(% control) |
Current
(nA) |
| 14±2.6 ** | 8±1.6 | 129±9.6 | 117±8.9 | 51±5.8 * | 101±8.1 | 29±2.3 |
| n=18 | n=4 | n=22 | n=22 | n=10 | n=6 |
Values in the table for each agonist or sensory response type are means of percentage of control +
standard error of the mean for n neurones. The mean iontophoretic current for LY367385 is
shown in the final column. Values marked with * or ** are significantly different from control
values (P<0.05, P<0.01: Wilcoxon signed rank test).
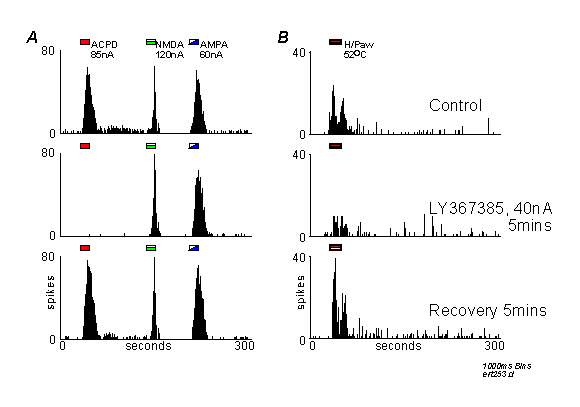
Peristimulus time histograms from a single nociceptive thalamic neurone responding to iontophoretic applications of ACPD, NMDA and AMPA (A) or to noxious stimulation of the contralateral hindpaw (H/Paw, B). Histograms show action potential spikes counted into 1000ms epochs ('bins'), and agonists/stimuli were presented as indicated by the markers above each record. In both A and B, upper records are controls, middle row of records are in the presence of LY367385 (ejected at 40nA prior to the start of the record), and lower records are recoveries commenced 5 minutes after the end of the antagonist ejection.
A: LY367385 antagonised the response to ACPD, but not the response to NMDA or AMPA.
B: LY367385 reduced the response to noxious thermal stimulation.
NMDA N-methyl-D-aspartate
AMPA (±)-a-amino-3-hydroxy-5-methyl-4-isoxazolepropionate
ACPD 1S,3R-aminocyclopentane-1,3-dicarboxylate
LY367385 S-2-methyl-4-carboxy-phenylglycine
DHPG S-3,5-dihydroxyphenylglycine
mGluR metabotropic glutamate receptor
REFERENCES
1. Abe T, Sugihara H, Nawa H, Shigemoto R, Mizuno N, and Nakanishi S. (1992) Molecular
characterization of a novel metabotropic glutamate receptor mGluR5 coupled to inositol
phosphate/Ca2+ signal transduction. J. Biol. Chem. 267, 13361-13368.
2. Aniksztejn L, Otani S, and Ben-Ari Y. (1992) Quisqualate metabotropic receptors modulate NMDA currents and facilitate induction of long-term potentiation through protein kinase C. Eur. J. Neuroscience 4, 500-505.
3. Charpak S, and Gähwiler BH. (1991) Glutamate mediates a slow synaptic response in hippocampal slice cultures. Proc. Royal Soc. London B: 243, 221-226.
4. Clark BP, Baker SR, Goldsworthy J, Harris JR, and Kingston AE. (1997) (+)-2-Methyl-4-carboxyphenylglycine (LY367385) selectively antagonises metabotropic glutamate mGluR1 receptors. Bioorg. Med. Chem. Letts. 7, 2777-2780.
5. Eaton SA, Birse EF, Wharton B, Sunter DC, Udvarhelyi PM, Watkins JC, and Salt TE (1993) Mediation of thalamic sensory responses in vivo by ACPD-activated excitatory amino acid receptors. Eur. J. Neuroscience 5, 186-189.
6. Eaton SA, and Salt TE. (1996) Role of NMDA and metabotropic glutamate receptors in cortico-thalamic excitatory post-synaptic potentials in vivo. Neurosci. 73, 1-5
7. Eaton SA, and Salt TE. (1990) Thalamic NMDA receptors and nociceptive sensory synaptic transmission. Neurosci. Letts. 110, 297-302.
8. Fitzjohn SM, Irving AJ, Palmer MJ, Harvey J, Lodge D, and Collingridge GL. (1996) Activation of group I mGluRs potentiates NMDA responses in rat hippocampal slices. Neurosci. Letts. 203, 211-213.
9. Godwin DW, Van Horn SC, Erisir A, Sesma M, Romano C, and Sherman SM. (1996) Ultrastructural localization suggests that retinal and cortical inputs access different metabotropic glutamate receptors in the lateral geniculate nucleus. J. Neurosci. 16, 8181-8192.
10. Kingston AE, Burnett JP, Mayne NG, and Lodge D. (1995) Pharmacological analysis of 4-carboxyphenylglycine derivatives: Comparison of effects on mGluR1Alpha and mGluR5a subtypes. Neuropharmacol. 34, 887-894.
11. Martin LJ, Blackstone CD, Huganir RL, and Price DL. (1992) Cellular localization of a metabotropic glutamate receptor in rat brain. Neuron 9, 259-270.
12. Masu M, Tanabe Y, Tsuchida K, Shigemoto R, and Nakanishi S. (1991) Sequence and expression of a metabotropic glutamate receptor. Nature 349, 760-765.
13. Mayer ML, Westbrook GL, and Guthrie PB. (1984) Voltage-dependent block by Mg2+ of NMDA responses in spinal cord neurones. Nature 309, 261-263.
14. McCormick DA, and Von Krosigk M. (1992) Corticothalamic activation modulates thalamic firing through glutamate "metabotropic" receptors. Proc. Nat. Acad. Sci. (USA) 89, 2774-2778.
15. Nakanishi S. (1992) Molecular diversity of glutamate receptors and implications for brain function. Science 258, 597-603.
16. Nowak L, Bregestovski P, Ascher P, Herbet A, and Prochiantz A. (1984) Magnesium gates glutamate-activated channels in mouse central neurones. Nature 307, 462-465 .
17. Peschanski M, Guilbaud G, Gautron M, and Besson JM. (1980) Encoding of noxious heat messages in neurons of the ventrobasal thalamic complex of the rat. Brain Res. 197, 401-413.
18. Pin J-P, and Duvoisin R. (1995) The metabotropic glutamate receptors: Structure and functions. Neuropharmacol. 34, 1-26.
19. Pisani A, Calabresi P, Centonze D, and Bernardi G. (1997) Enhancement of NMDA responses by group I metabotropic glutamate receptor activation in striatal neurones. Br. J. Pharmacol. 120, 1007-114.
20. Romano C, Van den Pol AN, and O'Malley KL. (1996) Enhanced early developmental expression of the metabotropic glutamate receptor mGluR5 in rat brain: Protein, mRNA splice variants, and regional distribution. J. Comp. Neurol. 367, 403-412.
21. Salt TE. (1987) Excitatory amino acid receptors and synaptic transmission in the rat ventrobasal thalamus. J. Physiol. 391, 499-510.
22. Salt TE, and Eaton SA. (1994) The function of metabotropic excitatory amino acid receptors in synaptic transmission in the thalamus: studies with novel phenylglycine antagonists. Neurochem. Int. 24, 451-458.
23. Salt TE, and Eaton SA. (1995) Modulation of sensory neurone excitatory and inhibitory responses in the ventrobasal thalamus by activation of metabotropic excitatory amino acid receptors. Neuropharmacol. 34, 1043-1051.
24. Shigemoto R, Nakanishi S, and Mizuno N (1992). Distribution of the mRNA for a metabotropic glutamate receptor (mGluR1) in the central nervous system: An in situ hybridization study in adult and developing rat. J. Comp. Neurol. 322, 121-135.
25. Turner JP, and Salt TE. (1996) Effect of metabotropic glutamate receptor activation on rat thalamocortical neurones in vitro. Soc. for Neurosci. Abstracts 22, 631.16.
26. Vidnyanszky Z, Goercs TJ, Negyessy L, Kuhn R, Knoepfel T, and Hamori J. (1996) Immunohistochemical visualization of the mGluR1a metabotropic glutamate receptor at synapses of corticothalamic terminals originating from area 17 of the rat. Eur. J. Neuroscience 8, 1061-1071.